Ready to Start?
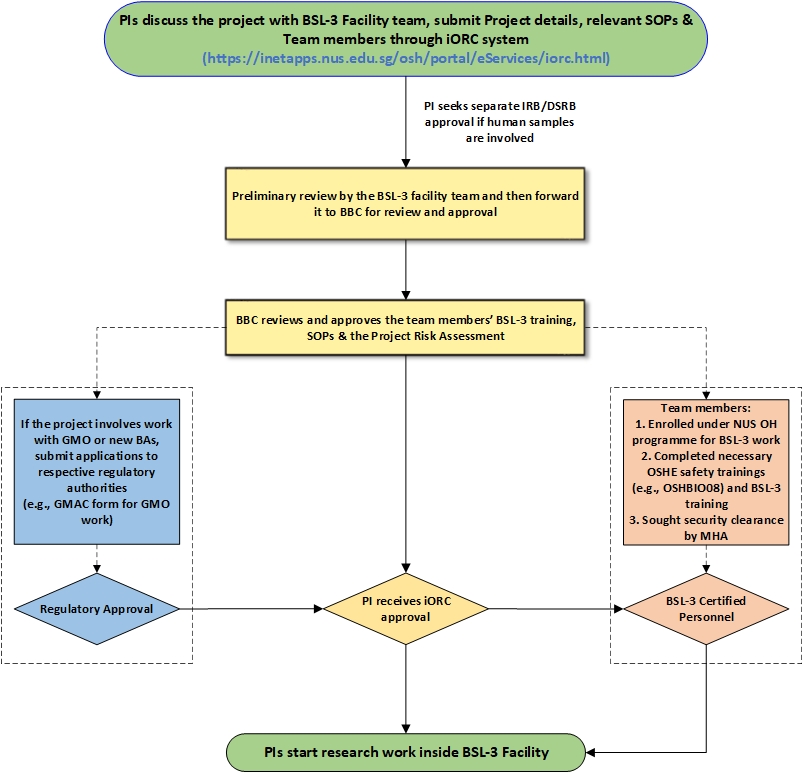
The Principal Investigators (PIs) who are starting new research projects in NUS Medicine BSL-3 Core Facility and subsequent amendments are required to conduct risk assessment to identify the safety and health risks associated with the project and then forward to BSL-3 Biosafety Committee (BBC) for review and approval on the online iORC system. BBC approves BSL-3 related research project risk assessments, regulatory applications, training applications for the new team members and relevant SOPs. If their project involves animal work, they would also need to submit IACUC forms. The BSL-3 Project Risk Assessment submission and approval process is detailed in the flow chart given below.
The research team members who are listed on PI’s iORC applications need to be trained according to the BSL-3 Training Programme and only the personnel who have completed the following requirements will be granted access into the BSL-3 Facility.
- Fit to work certificate issued by OH doctor.
- Personnel security clearance from MHA.
- Satisfactory completion of the BSL-3 training program and a pass the BSL-3 competency/certification assessment.