Gadsden T, Downey LE, Vilas VDR, Peiris D, Jan S. The impact of COVID-19 on essential health service provision for noncommunicable diseases in the South-East Asia region: A systematic review. The Lancet Regional Health - Southeast Asia. 2022;1:100010. https://doi.org/10.1016/j.lansea.2022.04.006.
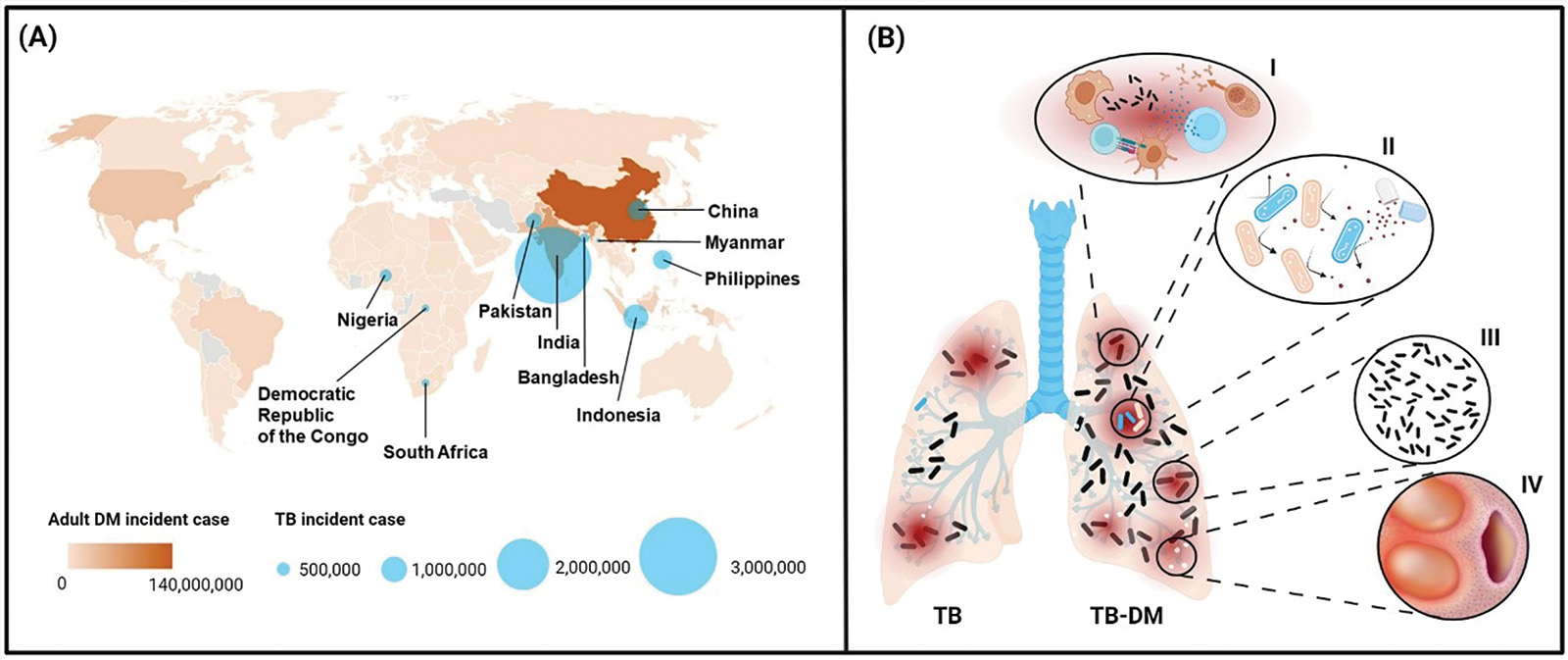
World Health Organisation. Global Tuberculosis Report 2021. Geneva, Switzerland: World Health Organisation; 2021.
World Health Organisation. Global Tuberculosis Report 2022. Geneva, Switzerland: World Health Organisation; 2022.
Gadsden T, Downey LE, Vilas VDR, Peiris D, Jan S. The impact of COVID-19 on essential health service provision for noncommunicable diseases in the South-East Asia region: A systematic review. The Lancet Regional Health - Southeast Asia. 2022;1:100010. https://doi.org/10.1016/j.lansea.2022.04.006.
Corona G, Pizzocaro A, Vena W, Rastrelli G, Semeraro F, Isidori AM, et al. Diabetes is most important cause for mortality in COVID-19 hospitalized patients: Systematic review and meta-analysis. Reviews in Endocrine and Metabolic Disorders. 2021;22(2):275-96. https://doi.org/10.1007/s11154-021-09630-8.
The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) - China, 2020. China CDC Wkly. 2020;2(8):113-22.
Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-22. https://doi.org/10.1016/S2213-8587(20)30272-2.
Bode B, Garrett V, Messler J, McFarland R, Crowe J, Booth R, et al. Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United States. Journal of Diabetes Science and Technology. 2020;14(4):813-21. https://doi.org/10.1177/1932296820924469.
Sen S, Chakraborty R, Kalita P, Pathak MP. Diabetes mellitus and COVID-19: Understanding the association in light of current evidence. World J Clin Cases. 2021;9(28):8327-39. https://doi.org/10.12998%2Fwjcc.v9.i28.8327.
Lim S, Bae JH, Kwon H-S, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nature Reviews Endocrinology. 2021;17(1):11-30. https://doi.org/10.1038/s41574-020-00435-4.
Zhang T, Mei Q, Zhang Z, Walline JH, Liu Y, Zhu H, et al. Risk for newly diagnosed diabetes after COVID-19: a systematic review and meta-analysis. BMC Med. 2022;20(1):444. https://doi.org/10.1186/s12916-022-02656-y.
Barrett CE, Koyama AK, Alvarez P, Chow W, Lundeen EA, Perrine CG, et al. Risk for Newly Diagnosed Diabetes >30 Days After SARS-CoV-2 Infection Among Persons Aged <18 Years - United States, March 1, 2020-June 28, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(2):59-65. https://doi.org/10.15585/mmwr.mm7102e2.
Li H, Tian S, Chen T, Cui Z, Shi N, Zhong X, et al. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes, Obesity and Metabolism. 2020;22(10):1897-906. https://doi.org/10.1111/dom.14099.
Gavkare AM, Nanaware N, Rayate AS, Mumbre S, Nagoba BS. COVID-19 associated diabetes mellitus: A review. World J Diabetes. 2022;13(9):729-37. https://doi.org/10.4239/wjd.v13.i9.729.
Groß R, Kleger A. COVID-19 and diabetes — where are we now? Nature Metabolism. 2022. https://doi.org/10.1038/s42255-022-00691-w.
Migliori GB, Thong PM, Alffenaar J-W, Denholm J, Tadolini M, Alyaquobi F, et al. Gauging the impact of the COVID-19 pandemic on tuberculosis services: a global study. European Respiratory Journal. 2021:2101786. https://doi.org/10.1183/13993003.01786-2021.
Cilloni L, Fu H, Vesga JF, Dowdy D, Pretorius C, Ahmedov S, et al. The potential impact of the COVID-19 pandemic on the tuberculosis epidemic a modelling analysis. eClinicalMedicine. 2020; 28 https://doi.org/10.1016/j.eclinm.2020.100603.
World Health Organisation. Global Tuberculosis Report 2022. Geneva, Switzerland: World Health Organisation; 2022.
Migliori GB, Alffenaar J-W, Denholm J. Tuberculosis and COVID-19 co-infection: description of the global cohort. European Respiratory Journal. 2022;59(3):2102538. https://doi.org/10.1183/13993003.02538-2021.
Petrone L, Petruccioli E, Vanini V, Cuzzi G, Gualano G, Vittozzi P, et al. Coinfection of tuberculosis and COVID-19 limits the ability to in vitro respond to SARS-CoV-2. Int J Infect Dis. 2021;113 Suppl 1:S82-s7. https://doi.org/10.1016/j.ijid.2021.02.090.
Riou C, du Bruyn E, Stek C, Daroowala R, Goliath RT, Abrahams F, et al. Relationship of SARS-CoV-2-specific CD4 response to COVID-19 severity and impact of HIV-1 and tuberculosis coinfection. J Clin Invest. 2021;131(12 https://doi.org/10.1172%2FJCI149125.
Pathak L, Gayan S, Pal B, Talukdar J, Bhuyan S, Sandhya S, et al. Coronavirus Activates an Altruistic Stem Cell-Mediated Defense Mechanism that Reactivates Dormant Tuberculosis: Implications in Coronavirus Disease 2019 Pandemic. The American Journal of Pathology. 2021;191(7):1255-68. https://doi.org/10.1016/j.ajpath.2021.03.011.
Antonio-Arques V, Franch-Nadal J, Caylà JA. Diabetes and tuberculosis: A syndemic complicated by COVID-19. Med Clin (Engl Ed). 2021;157(6):288-93. 343 https://doi.org/10.1016/j.medcli.2021.04.004.
Restrepo BI, Fisher-Hoch SP, Smith B, Jeon S, Rahbar MH, McCormick JB, et al. Mycobacterial clearance from sputum is delayed during the first phase of treatment in patients with diabetes. Am J Trop Med Hyg. 2008;79(4):541-4.
Kumar NP, Fukutani KF, Shruthi BS, Alves T, Silveira-Mattos PS, Rocha MS, et al. Persistent inflammation during anti-tuberculosis treatment with diabetes comorbidity. eLife. 2019;8:e46477. https://doi.org/10.7554/eLife.46477.
Kumar NP, Fukutani KF, Shruthi BS, Alves T, Silveira-Mattos PS, Rocha MS, et al. Persistent inflammation during anti-tuberculosis treatment with diabetes comorbidity. eLife. 2019;8:e46477. https://doi.org/10.7554/eLife.46477.
Kumar NP, Moideen K, Sivakumar S, Menon PA, Viswanathan V, Kornfeld H, et al. Tuberculosis-diabetes co-morbidity is characterized by heightened systemic levels of circulating angiogenic factors. The Journal of infection. 2017;74(1):10-21. https://doi.org/10.1016/j.jinf.2016.08.021.
Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Fay MP, Nutman TB, et al. Type 2 diabetes mellitus coincident with pulmonary tuberculosis is associated with heightened systemic type 1, type 17, and other proinflammatory cytokines. Ann Am Thorac Soc. 2013;10(5):441-9. https://doi.org/10.1513/annalsats.201305-112oc.
Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Fay MP, Nutman TB, et al. Type 2 diabetes mellitus coincident with pulmonary tuberculosis is associated with heightened systemic type 1, type 17, and other proinflammatory cytokines. Ann Am Thorac Soc. 2013;10(5):441-9. https://doi.org/10.1513/annalsats.201305-112oc.
Eckold C, Kumar V, Weiner 3rd J, Alisjahbana B, Riza A-L, Ronacher K, et al. Impact of intermediate hyperglycaemia as well as diabetes on immune dysfunction in tuberculosis. Clinical Infectious Diseases. 2020. https://doi.org/10.1093/cid/ciaa751.
Oehlers SH, Cronan MR, Scott NR, Thomas MI, Okuda KS, Walton EM, et al. Interception of host angiogenic signalling limits mycobacterial growth. Nature. 2015;517(7536):612-5. https://doi.org/10.1038/nature13967.
Miow QH, Vallejo AF, Wang Y, Hong JM, Bai C, Teo FSW, et al. Doxycycline host-directed therapy in human pulmonary tuberculosis. J Clin Invest. 2021;131(15) https://doi.org/10.1172/JCI141895.
Xu Y, Wang L, Zimmerman MD, Chen K-Y, Huang L, Fu D-J, et al. Matrix metalloproteinase inhibitors enhance the efficacy of frontline drugs against Mycobacterium tuberculosis. PLoS Pathog. 2018;14(4):e1006974-e. https://doi.org/10.1371/journal.ppat.1006974.
Pan SC, Ku CC, Kao D, Ezzati M, Fang CT, Lin HH. Effect of diabetes on tuberculosis control in 13 countries with high tuberculosis: a modelling study. Lancet Diabetes Endocrinol. 2015;3(5):323-30. https://doi.org/10.1016/s2213-8587(15)00042-x.