Giving a literal expression to the idiom, researchers from National University of Singapore (NUS) have uncovered the secret behind how blood cells release a vital biological substance involved in essential processes like immune and blood vessel functions. This knowledge of the pathway by which blood cells release sphingosine-1-phosphate (S1P) carries broad implications for the treatment of various immune and vascular diseases.
S1P is a lipid that is released into the circulation system and directs the movement of immune cells (such as T and B cells) as part of the body’s immune response. However, too much of these immune cells can cause conditions such as autoimmune and inflammatory diseases. Blocking the S1P signalling pathway with a drug called Fingolimod has been a successful approach in the treatment of multiple sclerosis, an autoimmune disease that affects nerve cells, the brain and spinal cord. On the other hand, too little S1P in the circulation is also harmful to blood vessels, often causing vascular complications such as cardiovascular diseases and stroke.
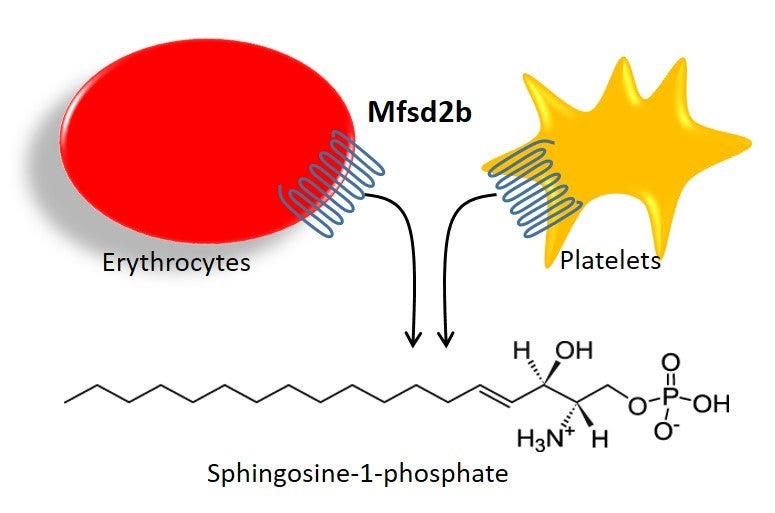
For a long time, researchers had suspected that red blood cells and platelets were the major suppliers of S1P to the blood. However, it was unclear how S1P was being transported into the body’s circulation system. “Our study identified a critical molecular player for S1P production,” said Dr Long Nguyen, who is an assistant professor from the Department of Biochemistry at the NUS Yong Loo Lin School of Medicine (NUS Medicine). “This opens up new avenues of investigation aimed at controlling the activity of S1P for the treatment of various diseases.” This study was published in Nature on 18 October 2017.
In the landmark study, Dr Nguyen’s team, including postdoctoral fellow Dr Thiet Vu, found that the absence of a transport protein namely Mfsd2b is related to low levels of S1P in the blood. This resulted in abnormally low numbers of T and B cells, and increased sensitivity to anaphylactic shock, a severe allergic reaction. Their breakthrough findings pave the way for the manipulation of S1P levels in the blood for the treatment of inflammatory and vascular diseases (see Appendix). The team also found that a lack of Mfsd2b is linked to low red blood cell counts, and chemotherapy and radiotherapy sensitivities. This suggests that increasing plasma S1P levels could be beneficial to cancer patients receiving chemotherapy and radiotherapy treatments.