At different points in the study, participants have a PET scan (or Positron Emission Tomography brain scan), to look at amyloid and tau (another protein) in their brain. This helps the participants and researchers track their health and this is the first study of its kind to use PET scans to measure amyloid and tau in all participants. The PET scan takes pictures of participants’ brains, allowing researchers to see and track changes in amyloid and tau levels.
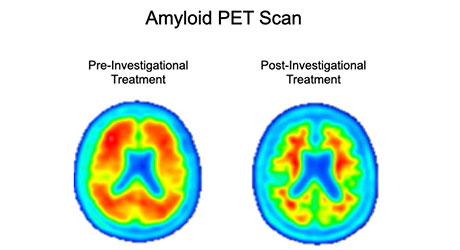
Amyloid PET imaging scans from a representative participant in the Phase 2 trial of BAN2401 (lecanemab) – the investigational treatment being tested in the AHEAD 3-45 Study. Amyloid PET scans measure the levels of amyloid plaque in the brain. The image on the left is taken before the participant has started on the investigational treatment. The image on the right is taken after 18 months of investigational treatment with BAN2401 (lecanemab), indicating a reduction of amyloid plaque burden in the brain. (Data presented at AAIC 2019)