IRF3 inhibits development of obesity and type 2 diabetes
Published: 10 Sep 2021
By Associate Professor Zhang Yongliang, Department of Microbiology and Immunology, NUS Yong Loo Lin School of Medicine
The global epidemic of obesity poses a major public health concern. Obesity, characterised by expansion of dysfunctional white adipose tissues (WATs) for storage of a surplus of energy due to excessive caloric intake, plays a major role in the development of chronic health conditions such as type 2 diabetes (T2D), fatty liver and cardiovascular diseases. WATs are mainly composed of fat cells (known as adipocytes) which play an important role in maintaining systemic metabolic homeostasis. In obesity, adipocytes are dysfunctional, leading to recruitment of inflammatory cells such as macrophages. These inflammatory macrophages produce soluble factors causing adipose tissue inflammation, leading to the development of metabolic disorders such as the loss of sensitivity to insulin, which is known as insulin resistance. Obesity and insulin resistance could further progress to type 2 diabetes (T2D). In our study published in Cell Death & Differentiation in June 2021, we discovered that a molecule called interferon regulatory factor 3 (IRF3) regulates the function of both adipoctyes and macrophages in WATs, thereby inhibiting the development of obesity and type 2 diabetes.
First, we found that in humans, IRF3 expression was negatively correlated with T2D. WATs from obese and diabetic patients had reduced expression of IRF3 compared to that in WATs from obese individuals without diabetes, suggesting that loss of IRF3 expression in WATs is associated with the progression of obesity-associated metabolic disorders. In T2D patients, higher expression of IRF3 in WATs was associated with better insulin sensitivity, whereas lower expression of IRF3 was associated with more severe disease. In animal models, high-fat-diet (HFD) feeding for eightweeks resulted in an increased expression of IRF3 in WATs, but not in skeletal muscle or liver. However, continuous feeding with HFD for 12-weeks resulted in loss of IRF3 expression in WATs, suggesting that loss of IRF3 expression in WATs is associated with development of more severe metabolic disease.
Next, we examined what the deficiency of IRF3 would cause, and results showed that it would lead to the development of obesity with abnormal weight gain, and expanded fat tissue containing larger adipocytes in the belly with ageing. The development of obesity caused by the deficiency of IRF3 was associated with the development of other metabolic disorders, including insulin resistance and glucose tolerance, two symptoms that are causatively linked with the development of type 2 diabetes. Indeed, these symptoms were further progressed to type 2 diabetes over time. Hence, our study thus far suggests that IRF3 has a protective role in the development of obesity and obesity-associated metabolic disorders. This molecule inhibits the development of obesity and helps to preserve the sensitivity of the body to insulin to regulate blood glucose level, thereby preventing the development of diabetes.
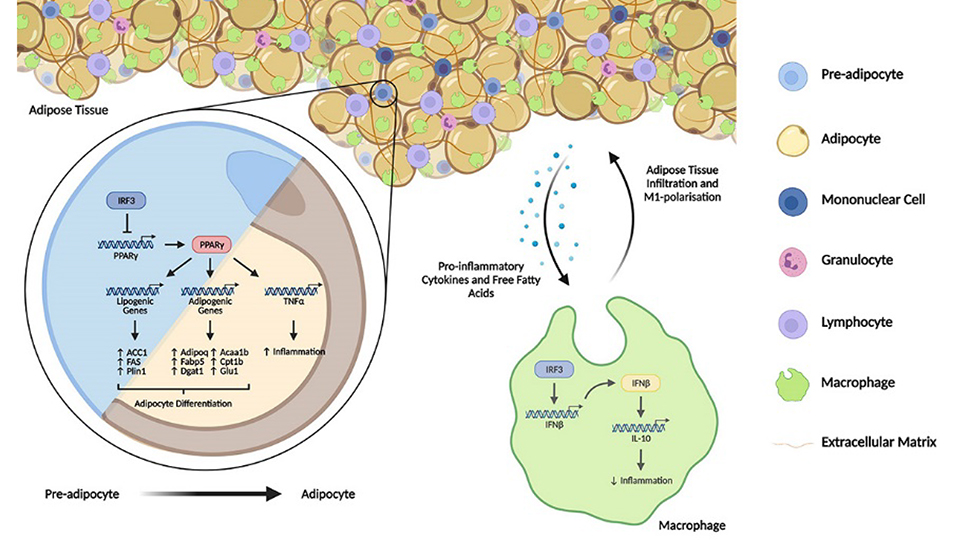
We further investigated the cellular and molecular mechanisms underlying the protective function of IRF3 in obesity and T2D. In adipocytes, we found that IRF3 controls the expression and function of a molecule called peroxisome proliferator-activated receptor gamma (PPARγ), which is a master regulator of adipocyte differentiation from its precursors called pre-adipocytes. Upon differentiation, adipocytes acquire its function such as responsiveness to insulin and accumulation of lipid droplets in cytoplasm for storage and later usage to supply energy. This process is called adipogenesis which requires the expression of PPARγ to induce its various target genes to form a gene programme to drive the differentiation. We found that in response to the induction for differentiation, the expression of PPARγ and its targeted-gene programme in preadipocytes were induced and gradually increased. Meanwhile the expression and activation of IRF3 were also induced and increased. IRF3 then controlled the magnitude of PPARγ and its targeted-gene programme thereby restraining the adipogenesis. As such, deficiency of IRF3 resulted in an earlier and increased expression of PPARγ and PPARγ–regulated adipogenesis gene programme. Consequently, the IRF3 deficient adipocytes were found to be functionally abnormal as shown by increased lipid accumulation and increased expression of molecules that are associated with development of insulin resistance and immune cell recruitment. Indeed, immune cells such as macrophages in WATs play important roles in adipose tissue function and systemic metabolic homeostasis. Adipose tissue macrophages are particularly important due to their ability to produce various inflammatory cytokines including TNFα, IL-6 and IL-1β which are able to directly cause insulin resistance. We observed increased macrophage infiltration in IRF3 deficient WATs compared to WT WATs. In addition, IRF3 deficient macrophages were found to be more inflammatory, capable of producing increased amounts of inflammatory cytokines including TNFα, IL-6 and IL-1β compared to WT cells. Consequently, the IRF3 deficient macrophages had higher ability to cause insulin resistance of adipocytes than WT macrophages. Further investigation revealed that the reason behind this increased inflammatory phenotype of IRF3 deficient macrophages was due to lack of IFNβ expression of the cells. Through the induction of an anti-inflammatory cytokine known as IL-10, IFNβ is required for the inhibition of the expression of various inflammatory cytokines in macrophages. Hence, deficiency of IRF3, the master regulator of IFNβ in macrophages, resulted in increased infiltration of macrophages into WATs and the IRF3 deficient macrophages produce increased amounts of inflammatory cytokines to cause adipose tissue inflammation, thereby contributing to the development of obesity-associated metabolic disorders such as insulin resistance.
In summary, our study, in collaboration with Professor Anthony Vidal-Puig at the University of Cambridge, demonstrated that the protective function of IRF3 against obesity and T2D requires its cooperative effects in multiple cellular compartments. In adipocytes, it regulates adipogenesis through a PPARγ-regulated adipogenic program, where its deficiency results in dysfunctional adipocytes. In adipose tissue macrophages, it is required for the suppression of inflammatory activation through the IFNβ-IL-10 axis, without which would increase adipose tissue inflammation during WAT expansion. Hence, this study provides us with a glimpse of how IRF3 can regulate metabolism to prevent the onset of obesity and its associated T2D. It also implies that IRF3 is a potential target for the development of therapeutic strategy for the prevention and treatment of obesity-associated diseases.

