Insights into functions of micronutrient transporters may pave way for new treatments for neurological diseases
Published: 28 May 2024
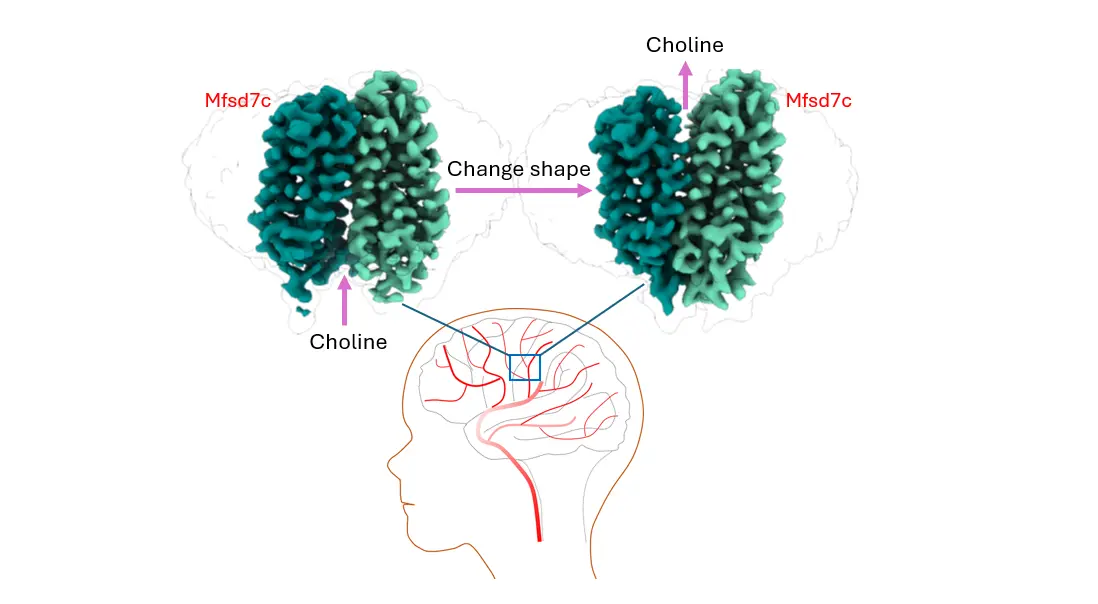
The molecular actions of Mfsd7c (also known as FLVCR2 in red labels) during choline transport at the blood brain barrier, excess choline in the brain is exported out by Mfsd7c. Photo Credits: Yong Loo Lin School of Medicine
The nutrients in our bodies help to provide daily energy and power the maintenance of cellular structure and function. But how do nutrients get distributed throughout the body in a specific manner?
Led by Associate Professor Nguyen Nam Long, scientists from the Immunology Translational Research Programme at the Yong Loo Lin School of Medicine, National University of Singapore (NUS Medicine) have discovered that Mfsd7c or FLVCR2 and Mfsd7b or FLVCR1 are the transporters for micronutrient choline and ethanolamine.
Human cells have a very limited capacity to synthesise these essential nutrients, which need to be derived from foods.
Working in collaboration with counterparts from the Max Planck Institute of Biophysics at Frankfurt, Germany, the NUS researchers expressed and purified these human proteins from mammalian cells to determine the atomic structures of these two protein transporters.
Cryo Electron Microscopy (Cryo-EM), an advanced imaging technique that helps to illuminate the three-dimensional structure of molecules at near-atomic resolution, was used to capture different angles of the architecture of these proteins during the transportation of micronutrient choline and ethanolamine. The team was then able to use molecular dynamics simulations to build working models for these transporters, which resembled their operations when transporting these micronutrients in real life.
At the molecular level, the research team observed that due to slight atomic structures, protein FLVCR1 prefers to deliver ethanolamine, which is an essential molecule that human cells must acquire from foods, while protein FLVCR2 has a higher affinity for transporting choline. Proteins FLVCR1 and FLVCR2 assist the cells to receive or secrete choline or ethanolamine, according to demand.
From the original intention of investigating how the mutations of proteins FLVCR1 and FLVCR2 causes different diseases, the research has now taken a different turn, prompting the team to take this knowledge a step further to understand how proteins FLVCR1 and FLVCR2 operate to transport these micronutrients through the cell membranes for absorption into the body.
Protein FLVCR2/MFSD7c has also been identified as a drug target for the treatment of Alzheimer’s disease because blocking its function at the blood-brain barrier can increase the level of choline for making more acetylcholine, a neurotransmitter that is reduced in patients with Alzheimer’s disease.
Understanding the intricacies of how nutrients are transported lays the foundation for drug development where small molecules or biologics can be designed to target the FLVCR2/MFSD7c function easily.
Titled ‘Molecular mechanism of choline and ethanolamine transport in humans’, the study was published in Nature on 22 May 2024.
Read the media release here for more details.

