
Issue 43 / August 2022
SCIENCE OF LIFE
Intraocular Lenses: Degree of Clarity

By Dr David Chen Ziyou, Clinical Lecturer, NUS Yong Loo Lin School of Medicine Associate Consultant, Department of Ophthalmology, National University Hospital
A clear-eyed look at the history of intraocular lenses.
A (random) walk down visionary lane
Sight is one of the five senses, and its importance to humans is perhaps best encapsulated by the cliché, ‘the eyes are the window to the soul’. Our vision constitutes our living reality, and its importance has been extolled extensively across different cultures.
Lenses are paramount in the function of vision and restoration of vision. From eyeglasses to intraocular lenses, there is a multitude of innovations which happened not by design, but by chance.
In this article, we take a brief walk down the evolution of lenses and how the recurring theme of serendipity exemplifies the Hummingbird effect and role of cross-pollination in innovative solutions for sight restoration. The history of glasses in general would first be covered in brief, followed by a deeper dive into the incidental discovery and subsequent explosion of breakthroughs in intraocular lens (IOL) technology due to innovations in adjacent industries or associated surgical techniques. Finally, we discuss the future of IOL development and how it attempts to defy the age-old axiom of senescence.
Lenses are paramount in the function of vision and restoration of vision. From eyeglasses to intraocular lenses, there is a multitude of innovations which happened not by design, but by chance.
A brief history of glasses—the original man-made lens
In the book by popular science author Steven Johnson, “How We Got to Now: Six Innovations That Made the Modern World”,1 Johnson famously described how the accidental creation of the ornamental crystal glass by Angelo Barovier in the 15th century led to the discovery of the light-bending property—known as refraction—of this clear material. At around the same time and place, Johannes Gutenberg invented the movable-typing printing press, which amplified the need for good reading vision and accelerated the popularisation of reading glasses. Johnson writes, ‘printing press created a surge in demand for spectacles, as the new practice of reading made Europeans across the continent suddenly realise that they were farsighted…[this] encouraged a growing number of people to produce and experiment with lenses.’ 1 These experimentations with lenses led to the invention of eyeglasses and microscopes which created the discipline of cellular biology, a phenomenon he coined the Hummingbird effect.
Eyeglasses are used to treat a variety of refractive errors, a medical condition where the focal point of light does not fall on the retina of the eye appropriately. Concave lenses are used to treat patients with myopia, or near-sightedness, where the light rays converge anterior to the retina (Figure 1); conversely, convex lenses are used to treat patients with hyperopia, or far-sightedness. In the 18th century, Benjamin Franklin, polymath and one of the Founding Fathers of the United States, developed the world’s first bifocal glasses—with a top segment correcting for myopia and a bottom half correcting for hyperopia—by sawing two pieces of glass together.2 He performed this due to the need to simultaneously see the facial expressions of people at distance clearly, while maintaining the ability to read scripts at handheld distance well.
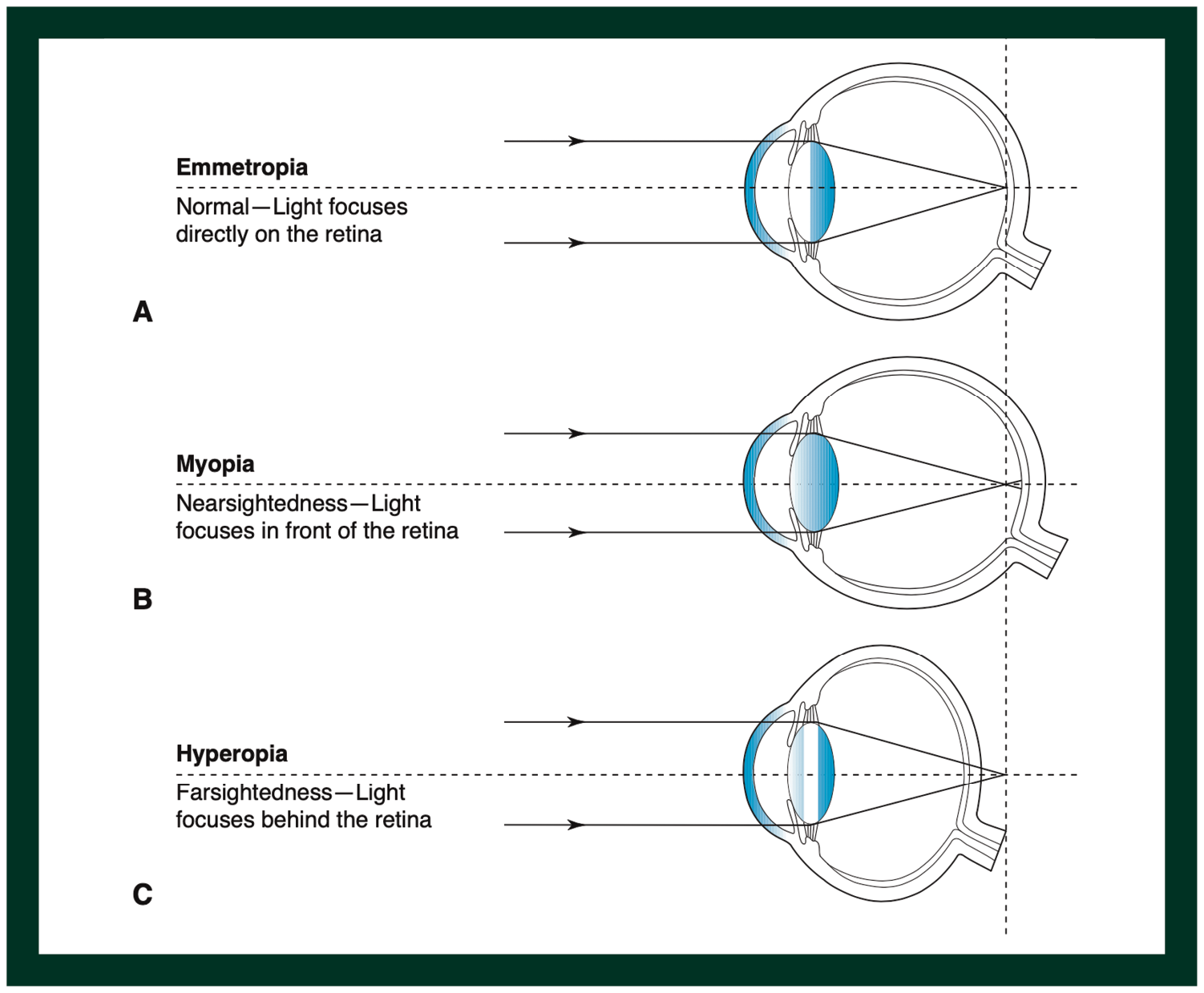
Figure 1. The position of light rays focusing, depending on different refractive statuses of the human eye. (Reproduced from Brodie SE et al, 2021 AAO Basic and Clinical Science Course Section 3: Clinical Optics)
Originally a medical device limited to the gentrified minority with resources to own them, eyeglasses have become progressively more accessible with the maturation of technology, making refractive error a correctable condition when it used to be debilitating to many. Today, the term ‘eyeglasses’ is perhaps even a misnomer at times; it is not necessarily made of glass (many are made of different polymers of plastic) and are sometimes worn as a fashion accessory (without optical correction properties), spurring a lucrative industry for fashion designers.3 This further exemplifies how innovation can take multiple shapes and forms.
Cataract—the (reversible) nemesis of sight
However, the invention of man-made lenses was long preceded by the natural refractive elements in the human body. Transparent solid elements in the human eye—namely, the cornea and crystalline lens—provide an au naturel solution to refractive correction. To put numbers in perspective, while eyeglasses typically cover a range of +6 dioptres (D) to -15D of correction (corresponding to 600 degrees of hyperopia and 1500 degrees of myopia respectively), the human cornea and crystalline lens provide a combined refractive power of more than 60D.4 The crystalline lens itself also has a shape-shifting property—known as accommodation—which allows it to change its refractive power on demand from ~20D to ~33D.5 This ability allows the human eye to see clearly from distance to near, provided the individual is emmetropic (Figure 1). If the person is myopic or hyperopic, eyeglasses which provide the accurate prescription could solve this problem, as explained earlier.
However, this ability is only valid insofar as the media involved in the optical pathway remains clear. Unfortunately, a cataract is a natural age-related degenerative condition, in which the crystalline lens becomes opacified and is no longer able to provide adequate transmission of light (Figure 2). This typically occurs when a person reaches the age of 50 years, and progressively worsens over many years leading to blindness if untreated. As life-expectancy improved from a mean of less than 30 years old in 1770 to more than 60 years old in 2001,6 the problem of reversible blindness from cataracts has become more prominent as the world ages and lives longer.
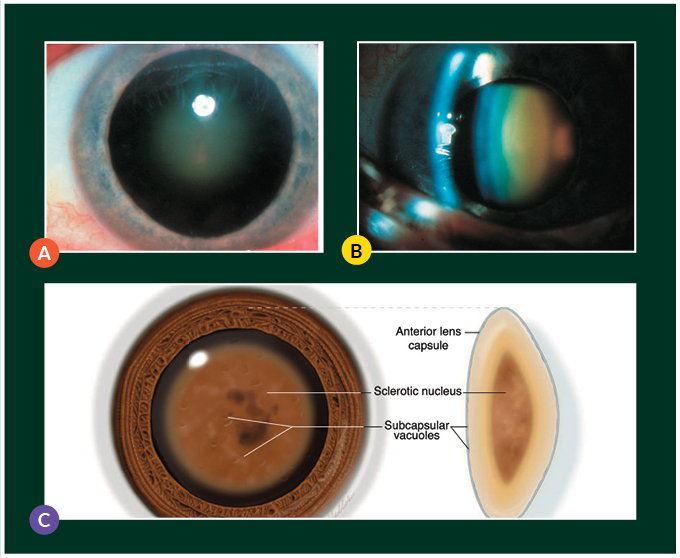
Figure 2. Clinical photograph of a nuclear sclerotic cataract on (A) diffuse illumination and (B) slit beam illumination, with (C) a schematic of a nuclear cataract. (Reproduced from Tsai LM et al, 2020 – 2021 AAO Basic and Clinical Science Course Section 3: Lens and Cataract)
The first cataract surgeries restored light but not sight
Cataract ‘surgery’ prior to the Renaissance era was a morbid affair. A technique known as couching was commonly the only ‘treatment’ available, where the ‘surgeon’ rapidly inserts a needle into the eye, pushing the cataract deep into the core of the eye (vitreous) and removing it from the visual axis. While this technique eliminates the media opacity, it effectively leaves the patient lens-less, or aphakic, thus about 20D hyperopic. This means the patient restores light, but not sight, and often at the expense of great pain and risks of severe infection (endophthalmitis).
In the 17th century, greater understanding of fine human anatomy (enhanced by the invention of the microscope) led to the refinements in surgical techniques, and Jacques Daviel (1696 – 1762) introduced a method to remove the cataract through a surgically-constructed 10–12mm corneal-limbal wound, a technique known as extracapsular cataract extraction (ECCE).7 This refined technique is more elegant than traumatically dislocating the cataract into the eye through couching. ECCE resulted in improved surgical safety (reduced infection and discomfort), but still only restored light, not sight.
As life-expectancy improved from a mean of less than 30 years old in 1770 to more than 60 years old in 2001, the problem of reversible blindness from cataract has become more prominent as the world ages and lives longer.
Several centuries then ensued with no significant improvements in visual outcomes, as there were no lenses available to be placed after cataract surgery, and patients remained aphakic—and therefore severely hyperopic—after surgery. To see better, patients had to wear thick aphakic eyeglasses, which are heavy and provided severely magnified and distorted vision.
The accidental discovery of the intraocular lens
The invention of the intraocular lens (IOL) was a serendipitous accident. Harold Ridley, an English ophthalmologist, was working as a medic with the Royal Air Force during World War II. On 15 August 1940, he received and treated a fighter pilot Gordon Cleaver, whose aircraft had been hit by cannon shells during combat over Winchester.8 Cleaver’s eyes sustained traumatic injuries from the Perspex (polymethylmethacrylate, PMMA) of the shattered cockpit of his Hurricane aircraft, and Ridley performed several emergency eye operations to restore his vision. While Cleaver only regained partial vision in one eye and was blinded in the other, Ridley discovered that PMMA did not induce inflammation in the eye.8

Figure 3. Photograph of the original Ridley lens, which was implanted in November 1949 – note how its biconvex design closely mimics that of the human eye. (Reproduced from Tsai LM et al, 2020 – 2021 AAO Basic and Clinical Science Course Section 3: Lens and Cataract)
Inspired by his discovery, Ridley proceeded to find manufacturers to produce a PMMA IOL and performed his first ECCE with IOL implantation on 29 November 1949 at St Thomas Hospital, London, on a 45-year-old woman (Figure 3). Due to the surgical challenges of this technique, complication rates were high initially, including glaucoma, uveitis, and dislocation.7 Ridley’s work was considered highly unconventional during his time, and he faced significant backlash from the medical community, though he was eventually recognised for his important contributions to cataract surgery and awarded a knighthood in February 2000.9
In the 1990s, Ridley received bilateral cataract surgery and IOL implantation at the same hospital where, 40 years ago, he stumbled on his seminal discovery.10 Interestingly, the fighter pilot Gordon Cleaver who had contributed to Ridley’s finding also underwent successful cataract surgery with an IOL implant in the 1980s, made from a material none other than PMMA. This time, it was intentionally and appropriately placed in his eye to restore vision.9
A visit to the dentist and the unexpected inspiration behind phacoemulsification
Sir Harold Ridley is not the only unconventional ophthalmologist in recent times. Since his invention of IOLs, cataract surgery was still limited by significant morbidity and inflammation due to the relatively large, imprecise 12 mm wounds created through ECCE, with some patients having to rest in hospital for up to two weeks.
In 1964, frustrated by the limitations of ECCE, Charles Kelman, a New York-based ophthalmologist, applied for a $299,000 research grant from the John A Hartford foundation to develop ‘a method for removing a cataract through an incision small enough so that no hospitali[s]ation will be required’.11 His initial ideas on creating smaller-wound cataract surgeries were unsuccessful, and repeated experiments on cats were futile. As chance would have it, six months before his grant funding ran out, Kelman visited his dentist for tartar removal, and experienced the newly introduced ultrasound probe (US3380446A, patent filed 3 September 1965).12 Kelman, a jazz musician, was intrigued by the high-pitched buzz of the ultrasonic probe, and thus the idea of using ultrasound to pulverise the crystalline lens was born. In 1967, he filed his own patent on a novel ultrasound probe for a technique that would become the standard of cataract surgery today, phacoemulsification (US3589363A, patent filed 25 July 1967).13
Though he published his technique describing how phacoemulsification allowed incisions as small as 3 mm to be used for cataract surgery in 1967,14 his contemporaries were not convinced. He faced significant criticism and skepticism in the 1970s, with some critics calling the procedure ‘ridiculous’.11,15 In 1973, some prominent ophthalmologists, perhaps concerned that they were losing their cataract patients to surgeons who practiced newer techniques, convinced the National Eye Institute to declare phacoemulsification experimental and not reimbursable through Medicare, the national insurance of the United States.16 Kelman, however, eventually proved his critics wrong, and he had IOL innovation to thank for that.
The Hummingbird effect of phacoemulsification on intraocular lens innovation, and vice versa
Prior to phacoemulsification, IOL development had stalled after Sir Ridley’s original invention for several decades. This was primarily because the bottleneck of vision restoration was not IOL design, but the unpredictability of wound sizes. With a 12 mm wound from ECCE, refractive outcomes were imprecise due to the surgically induced astigmatism which could be as high as 8D, and patients typically would expect a variability of at least 2D of refractive error no matter which IOL was chosen.
True accommodative IOLs remain an elusive goal and a problem yet to be solved. Current attempts at accommodative IOLs have proven to have limited success and true accommodation remains the holy grail of cataract surgery.
The advent of phacoemulsification greatly reduced wound sizes. Phacoemulsification, however, had its own existential crisis initially because no IOLs could fit through a 3 mm wound at the time of its invention! As a result, surgeons then had to manually open the wound to 6 mm for the IOL insertion.17 While this improved the visual outcomes as compared to ECCE, overall vision and predictability were still poor. The indirect beneficiary of phacoemulsification, however, was the renewed interest in IOL innovation, and various surgeons began experimenting with newer IOL designs.
In 1982, Thomas Mazzocco invented the first foldable IOL made of silicone (CA1275351C, priority date 5 February 1982).18,19 Using a plate design, this IOL could be inserted through a 3 mm wound, meaning surgeons no longer had to expand the typical phacoemulsification wound for IOL insertion. This, together with improved surgical techniques, intraocular materials and viscoelastic devices, catapulted phacoemulsification into the mainstream. Wound sizes continued to reduce (as small as 1.8 mm incisions are possible) while visual outcomes and refractive predictability continued to improve (≥80% of patients achieve within 0.5D of refractive target).20 This led to a flurry of new lenses which provided further improvements in visual quality beyond simple visual acuity (e.g. toric lenses which correct astigmatism, apheric IOL surfaces which reduce visual aberrations, etc).
Today, a healthy patient undergoing cataract surgery could expect distance vision equivalent to that of a healthy 20-year-old. Figure 4 summarises the timeline and close interlink between cataract surgery development and IOL development in recent times.
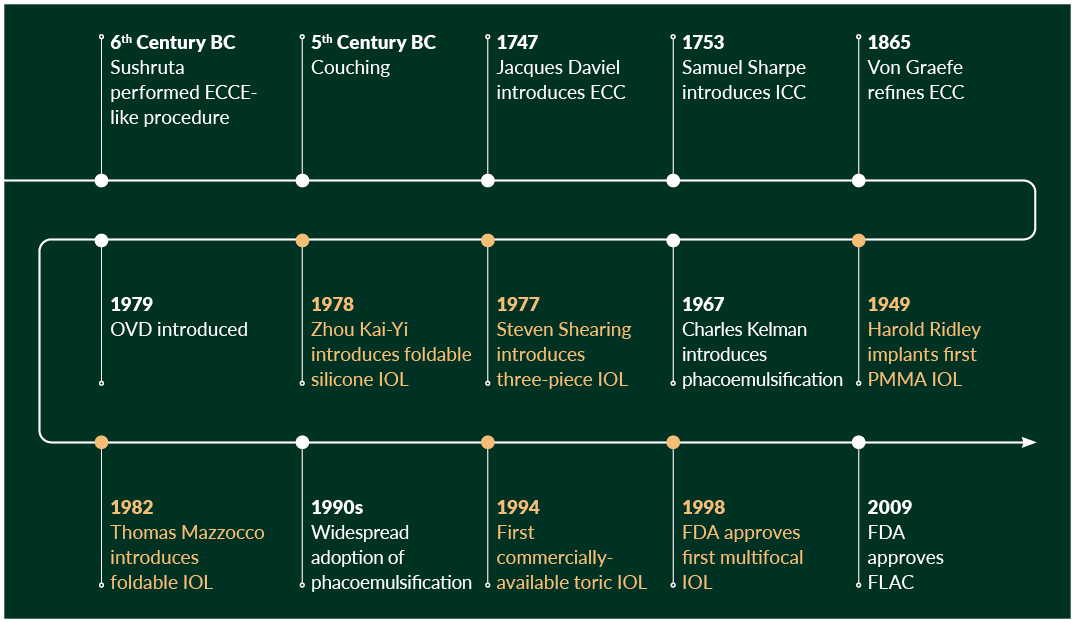
Figure 4. Timeline of cataract surgery development (white line) and intraocular lens development (orange line). ECCE, extracapsular cataract extraction; FDA, Food and Drug Administration; FLACS, femtosecond laser-assisted cataract surgery; ICCE, intracapsular cataract extraction; IOL, intraocular lens; OVD, ophthalmic viscoelastic devices; PMMA, poly(methylmethacrylate)
Presbyopia—the next hurdle and the future of intraocular lens innovation
IOLs have continued to improve with each iteration. Today, cataract surgery restores both light and sight. There is yet one problem, however, which has not been adequately addressed.
Presbyopia refers to the loss of accommodation over time, meaning a person loses his or her natural near reading ability from around 40 years old.7 With improved life expectancy and literacy, functional performance and visual expectations after cataract surgery have increased as well. Spectacle-independence has become a need instead of a want especially in the mask-wearing COVID-19 era.
Thus far, lens makers have made use of different optical principles to achieve pseudo-accommodation, creating a category of IOLs called multifocal lenses.7 These multifocal IOLs either bend or split light into different focal points, creating distinct zones of sharp vision—typically, distance, intermediate, and near reading. Two current leading concepts have improvised on light principles discovered centuries ago: the principle of refraction (Figure 5A) and the principle of diffraction (Figure 5B). When Dutch mathematician Willebrod Snel van Royen discovered the law of refraction in the early 1600s and English physicist Thomas Young described his double-slit experiment in 1801, they could hardly have imagined that their discoveries would one day find their way into implants to correct vision.
True accommodative IOLs, however, remain an elusive goal and a problem yet to be solved. Current attempts at accommodative IOLs have proven to have limited success and true accommodation remains the holy grail of cataract surgery.
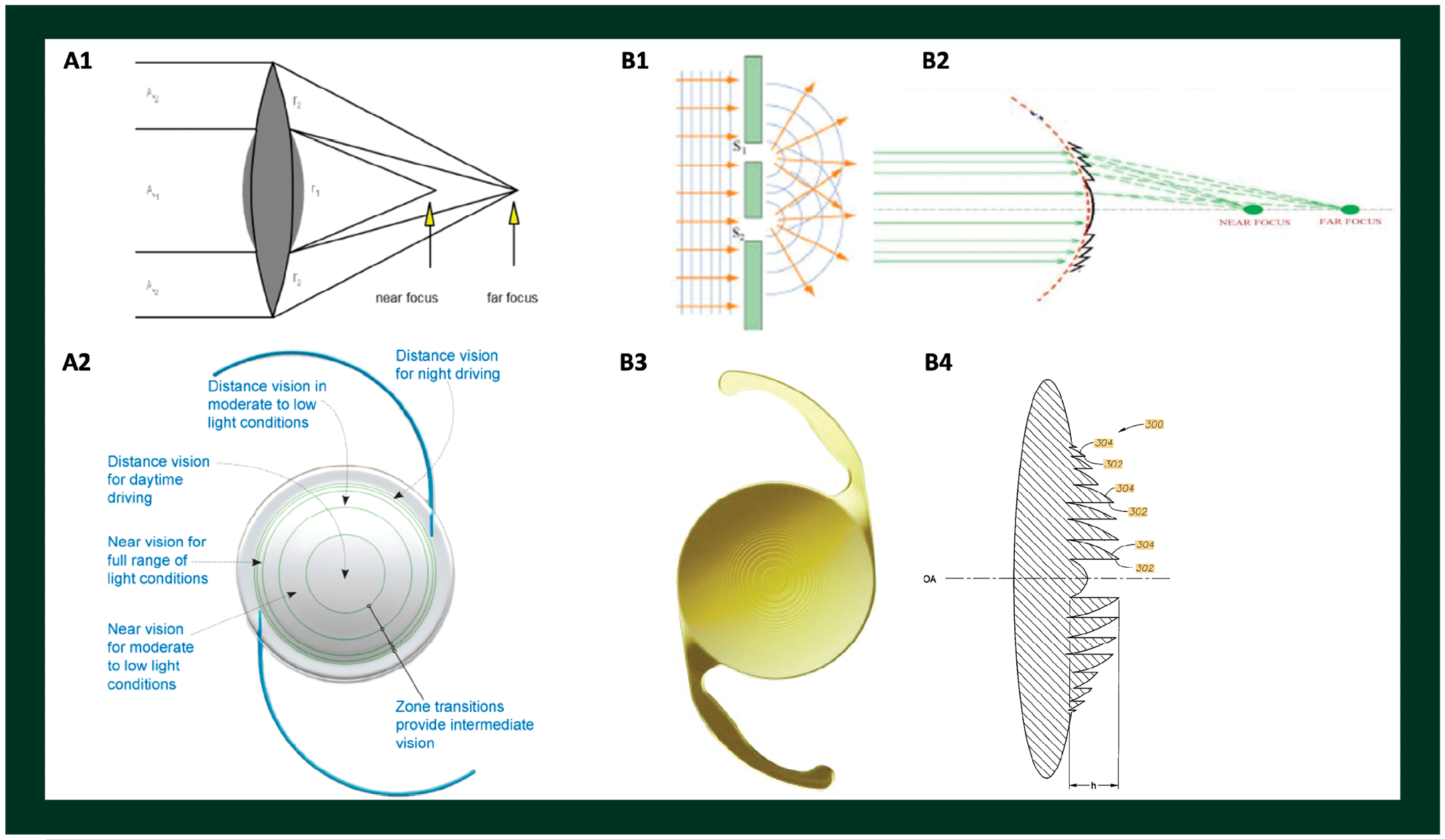
Figure 5. Examples of two designs of multifocal intraocular lenses based on different principles. (A1) Refractive design, where lenses have different zones of refractive indices bending light to distinct foci; (A2) an intraocular lens with refractive design; (B1) diffractive principles based on Young’s double-slit experiment, and (B2) diffractive design incorporated onto a lens where different constructive and destructive interferences provide blending zones of clear vision; (B3) and (B4) showing an example of diffractive intraocular lens in en face and cross-sectional view, respectively.
Lessons from the journey of intraocular lens discovery
This random walk down visionary lane has illustrated that the journey of innovation is replete with accidental discoveries, sometimes literally so. From the tragic salvation of Gordon Cleaver by Sir Harold Ridley to the million-dollar trip to his dentist by Charles Kelman, inspiration can strike anytime, anywhere. It is amazing how far cataract surgery has come from what was essentially brutally invasive trauma to the patient and which merely restored light but not sight to an elegant bloodless procedure which (almost) perfects vision beyond senescence. Biologists would be familiar with the anti-ageing role of telomerases, and cataract surgery increasingly resembles the age-defying telomerase of lens ageing. The elderly who would naturally be blind are seeing better than ever before, thanks to the culmination of innovations in IOL technology. Several lessons can be learnt from this meandering journey.
In a world where efficiency maximisation and opportunity cost-optimisation continue to dominate headlines and performance milestones, it is worth remembering that just like the best lessons, moments of inspiration are often caught, not taught.
Firstly, beyond the charm of Hummingbird effects—from the unexpected innovations in eyeglasses to the discovery of IOL catalysing small-wound cataract surgery, to the continual improvements in IOL precision when phacoemulsification enabled small-incision cataract surgery—it is evident that discoveries made today may only realise their full potential decades down the road. In the case of Snel’s discovery of refraction and Young’s discovery of diffraction, it may even ring true centuries down the road.
Next, besides mimicking the flight of insects to get nectar from flowers,21 hummingbirds are also responsible for another important analogy in innovation—cross pollination. Applicable technologies are disease- and domain-agnostic, and often find ways of application in parallel but distinct fields. High-frequency ultrasound-based technology do not only scale teeth, but also emulsify cataracts, and provide non-invasive imaging modality used throughout medical practice, most recently in super-resolution vascular oncological imaging.22
Finally, the path of innovators is often fraught with detractors. Both Sir Ridley and Dr Kelman faced their fair share of naysayers who continually discouraged their innovations, sometimes for cynical reasons. It takes a certain fortitude and can-do spirit in a lonely journey as the underdog who persistently strives against Goliaths of the day. Nonetheless, so long as the pursuit is for a genuine, unsolved clinical need, the persistence would often pay off eventually.
In a world where efficiency maximisation and opportunity cost-optimisation continue to dominate headlines and performance milestones, it is worth remembering that just like the best lessons, moments of inspiration are often caught, not taught.
Therefore, do not fret the more scenic path, for you never know what you might chance upon.
-
Johnson S. How we got to now: six innovations that made the modern world. New York: Riverhead Books, a member of Penguin Group (USA); 2014.
-
Bronowski J, British Broadcasting Corporation. The ascent of man [Internet]. London; New York: BBC Books Parkwest; 1990 [cited 2022 Feb 8]. Available from: https://archive.org/details/ascentofman0000bron_y1z2.
-
Pullin G. Design meets disability. Cambridge, Mass: MIT Press; 2009.
-
Elkington AR, Frank HJ, Greaney MJ. Clinical optics. 3. ed., reprinted. Oxford: Blackwell Science; 2006.
-
Optical Properties of the Eye – American Academy of Ophthalmology [Internet]. [cited 2022 Feb 8];Available from: https://www.aao.org/munnerlyn-laser-surgery-center/optical-properties-of-eye.
-
Riley JC. Estimates of Regional and Global Life Expectancy, 1800-2001. Population & Development Review 2005;31(3):537–43.
-
Tsai LM. 2019-2020 Basic and Clinical Science Course, Section 11 Lens and Cataract. [Internet]. S.l.: AMER ACADEMY OF OPHTHALMO; 2019 [cited 2022 Feb 8]. Available from: http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=2355040.
-
Lam A. Saving sight: an eye surgeon’s look at life behind the mask and the heroes who changed the way we see. First edition. Bokeelia, Florida: Irie Books; 2013.
-
Apple DJ. Sir Harold Ridley and his fight for sight: he changed the world so that we may better see it. Thorofare, NJ: Slack; 2006.
-
Williams HP. Sir Harold Ridley’s vision. Br J Ophthalmo 2001;85(9):1022–3.
-
Goes FJ, De Laey JJ. The eye in history. First edition. New Delhi: Jaypee-Highlights Medical Publishers, Inc; 2013.
-
Martin LG. Dental applicator [Internet]. 1968 [cited 2022 Feb 9];Available from: https://patents.google.com/patent/US3380446A/en?q=ultrasound+dental+scaling&sort=old.
-
Banko A, Kelman CD. Material removal apparatus and method employing high frequency vibrations [Internet]. 1971 [cited 2022 Feb 9];Available from: https://patents.google.com/patent/US3589363A/en?inventor=Charles+D+Kelman&sort=old.
-
Kelman CD. Phaco-emulsification and aspiration. A new technique of cataract removal. A preliminary report. Am J Ophthalmol 1967;64(1):23–35.
-
Kelman CD. Through my eyes: the story of a surgeon who dared to take on the medical world. 1st ed. New York: Crown Publishers; 1985.
-
Cataract Surgery and IOLs [Internet]. CRSToday. [cited 2022 Feb 9];Available from: https://crstoday.com/articles/2006-jan/crst0106_06-html/.
-
Obstbaum SA. Charles D. Kelman, MD (1930-2004). Arch Ophthalmol 2005;123(2):287.
-
Mazzocco TR. Early clinical experience with elastic lens implants. Trans Ophthalmol Soc U K 1985;104 ( Pt 5):578–9.
-
Mazzocco TR. Deformable intraocular lens structures and methods and devices for implantation [Internet]. 1990 [cited 2022 Feb 9];Available from: https://patents.google.com/patent/CA1275351C/en?assignee=thomas+mazzocco.
-
Stanojcic N, OʼBrart D, Hull C, et al. Visual and refractive outcomes and glistenings occurrence after implantation of 2 hydrophobic acrylic aspheric monofocal IOLs. J Cataract Refract Surg 2020;46(7):986–94.
-
Hurlbert AH, Hosoi SA, Temeles EJ, Ewald PW. Mobility of Impatiens capensis flowers: effect on pollen deposition and hummingbird foraging. Oecologia 1996;105(2):243–6.
-
Schmitz G, Dencks S. Ultrasound Imaging. Recent Results Cancer Res 2020;216:135–54.




