Synaptic Plasticity & Memory Lab
Research
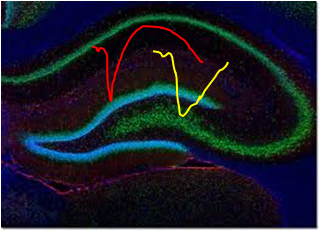
How does one acquire, retain, and retrieve memories? This fundamental question in neuroscience has intrigued many, including our team. Utilising electrophysiological, behavioural and molecular approaches, our lab studies the cellular basis of long-term memory (LTM) with the overall goal of unravelling of how the brain learns, remembers, recalls and forgets.
Working on brain regions associated with memory encoding, we focus on elucidating the formation of LTM. We also examine how synaptic tagging and capture, a conceptual framework for associative learning and LTM formation, is manifested neurally in different stages of physiology and disease. Lastly, synaptic populations do not exist in isolation and interact with one another. We are interested to observe these interactions and its effects on plasticity. These effects, known as metaplasticity, could be a compensatory mechanism that can transform otherwise transient memories to LTM. In all, these different avenues of research can encapsulate the diversity of synaptic interactions that confers us the ability to learn, forget and remember.
Synaptic Tagging and Capture: Making Memories Last
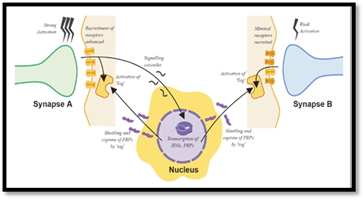
A schematic illustrating the mechanism of STC (Made using BioRender)

The first-ever comprehensive handbook for the Synaptic Tagging and Capture hypothesis – do check it out!
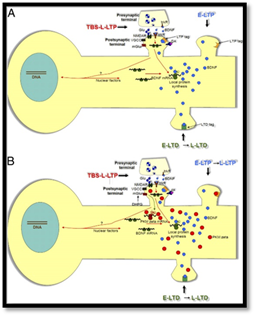
Different molecular players can play a role in how STC is expressed and compartmentalized across the neuron. (Adapted from Sajikumar & Korte PNAS, 2011)
Seemingly transient memories can withstand decay when it occurs in close temporal proximity to strong memory. This can be explained by the Synaptic Tagging and Capture hypothesis (STC), a conceptual framework that illustrates interactions of synapses in a neuronal population. This framework has been verified via electrophysiological, molecular, and behavioural approaches and remain a robust and versatile concept to explain associative learning. Thus, our team is interested to observe the different mechanisms of STC in health and disease, and its manifestations across different neuronal populations in the brain.
Unravelling the epigenetic influence on memory in health and aging
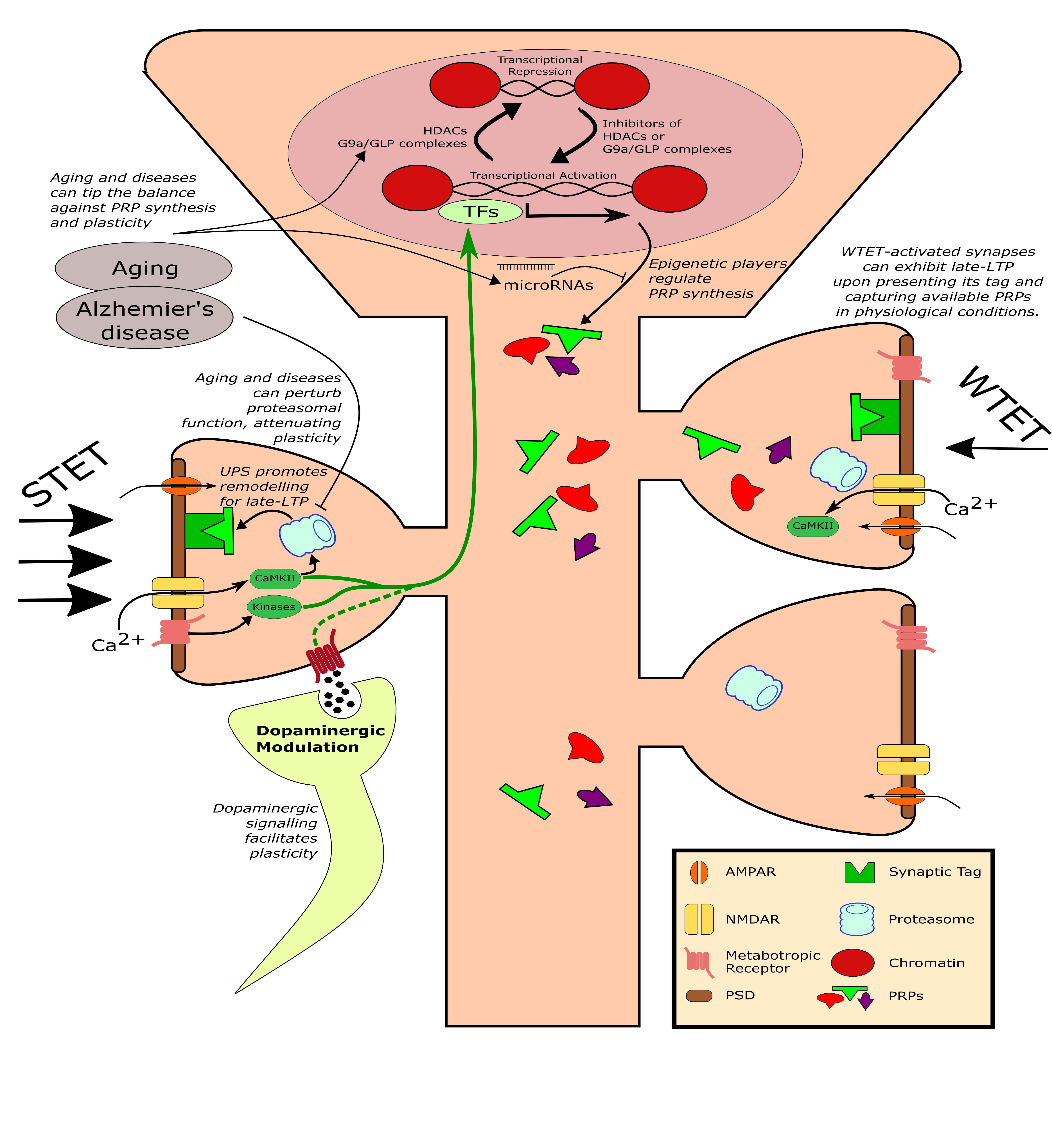
Different molecular mechanisms can influence plasticity and memory. (Adapted from Bin Ibrahim et al. FEBS J (2021))
Epigenetic-mediated gene regulation is vital for modulating activity-dependent plasticity. Firstly, histone acetyl transferases, histone methyltransferase and histone deacetylases in chromatin structure modification has been implicated in the transcriptional regulation of memory formation. Beyond the chromatin, MicroRNAs can exert post-transcriptional effects on a more synapse-specific level. As aging progresses, changes in epigenetics can be observed, which may dampen synaptic plasticity. Moreover, many of the neurodegenerative disorders including Alzheimer's disease (AD) are associated with pathogenic alteration in epigenetic factors leading to synaptic failure and cognitive decline.
Thus, we would like the probe the mechanisms of Aβ-dependent memory impairment in AD models via pharmacological approaches affecting these epigenetic regulators. The emerging field of neuroepigenetics might provide a promising therapeutic approach to counteract AD and other ageing related forms of memory decline.
Adequate sleep for a long healthspan
Sleep is essential for establishing long-term memory, thus sleep deprivation (SD) leads to major memory and cognitive defects. Thus, we seek to unravel the molecular mechanisms behind this phenomenon. One key molecular player is the p75 neurotrophin receptor, where its suppression leads to alleviation of SD-induced memory impairments.
Interestingly, p75 has also been implicated in AD and other age-associated diseases. Ageing is another biological phenomenon that increases disease susceptibility with the passage of time. By elucidating the downstream effects of p75 in ageing and SD, we can identify common pathways and trends that can provide therapeutic insights into extending healthspan as well as ameliorating SD effects.
CA2, a new area to be unfolded
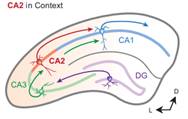
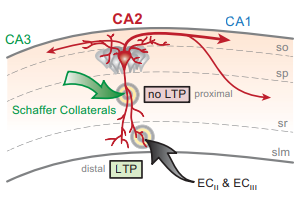
Neurons in hippocampal area CA2 exhibit unique plastic properties. (Adapted from Caruana et al., 2012)
doi: 10.1101/lm.025304.111
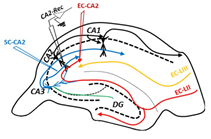
Investigation of CA2 synaptic activity requires electrode placement on 2 separate pathways. (Adapted from Dasgupta et al. eLife, 2020)
In the hippocampus, the small subregion CA2 has been largely overshadowed by the CA3 and CA1. However, recent research has invigorated interest in CA2 and its role in social memory.
Additionally, CA2 has many distinct biochemical and electrophysiological properties than its well-known CA1 and CA3 counterparts. Furthermore, its relative resilience in temporal lobe epilepsy and relative susceptibility in neuropsychiatric disorders may provide insights to disease progression.
In all, this set of unique properties begs the question of how this distinction leads to differentiated encoding of social memory and modulation of other hippocampal function. As such, we aim to understand the various aspects of associative plasticity in CA2 and how modulation of plasticity in CA2 by various plasticity regulators can dynamically regulate the plasticity in CA1, the major output pathway of memory formation.
Stress: Good or bad or both?
In the environment, stress plays a role in shaping our learning and behaviour, and has a hormetic effect on our behavioural, mental and physiological well-being. Thus, dissecting its various forms and manifestations will be vital in understanding its diverse effects.
Acute stress, defined as a psychological response to a negative experience lasting less than a week, has been implicated in memory encoding. Yet, studies are often contradictory, suggesting either a negative or positive effect on memory. Hence there is a growing need to understand how acute stress influences the mechanism behind learning and memory to provide a clear picture of its effects. Plugging this gap of knowledge may aid in understanding how we recall events upon exposure to acute stressors.
Besides that, stress can have different effects on separate age groups. Exposure to either prenatal or early post-natal period stress impacts developmental pathways. This can lead to lasting regulatory and structural changes that predisposes adulthood to diseases. Experimental, epidemiological and clinical studies have established that early-life stress results in long-term hyper-responsiveness. This, in addition to an exaggerated level of circulating glucocorticoids leads to anxiety, depression-like behaviours and post-traumatic stress disorders.
New perspectives in Alzheimer’s disease and aging
The brain with Alzheimer’s. (From Wikipedia)
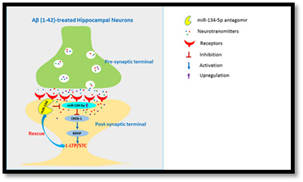
Epigenetic players such as microRNA-134-5p are implicated in Alzheimer’s disease. (Graphical abstract from Baby et al. Aging Cell, 2019)
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by the loss of memory and cognition, and it is the most common type of dementia in the world. Although there are two main hypothesis as the cause of the disease, β-amyloid plaques and Tau fibrillary tangles, drugs designed to tackle any of the two have proven non-efficient in clinical trials. Therefore a more comprehensive understanding of the disease is needed.
In our lab we study the differences between male and females in AD, as it has been shown that the risk is higher and the disease progresses faster in females. Additionally, we study the role of Nogo-A in AD pathology, as it has been recently shown to restrict plasticity in healthy animals and has been proven to be overexpressed in AD hippocampus. Lastly, we also investigate the role of different subcellular players such as the proteasome and epigenetic mechanisms in AD progression. With a greater appreciation of the mechanistic processes behind AD, we aim to seek possible therapeutic routes for disease amelioration and preventive strategies to promote healthy aging.