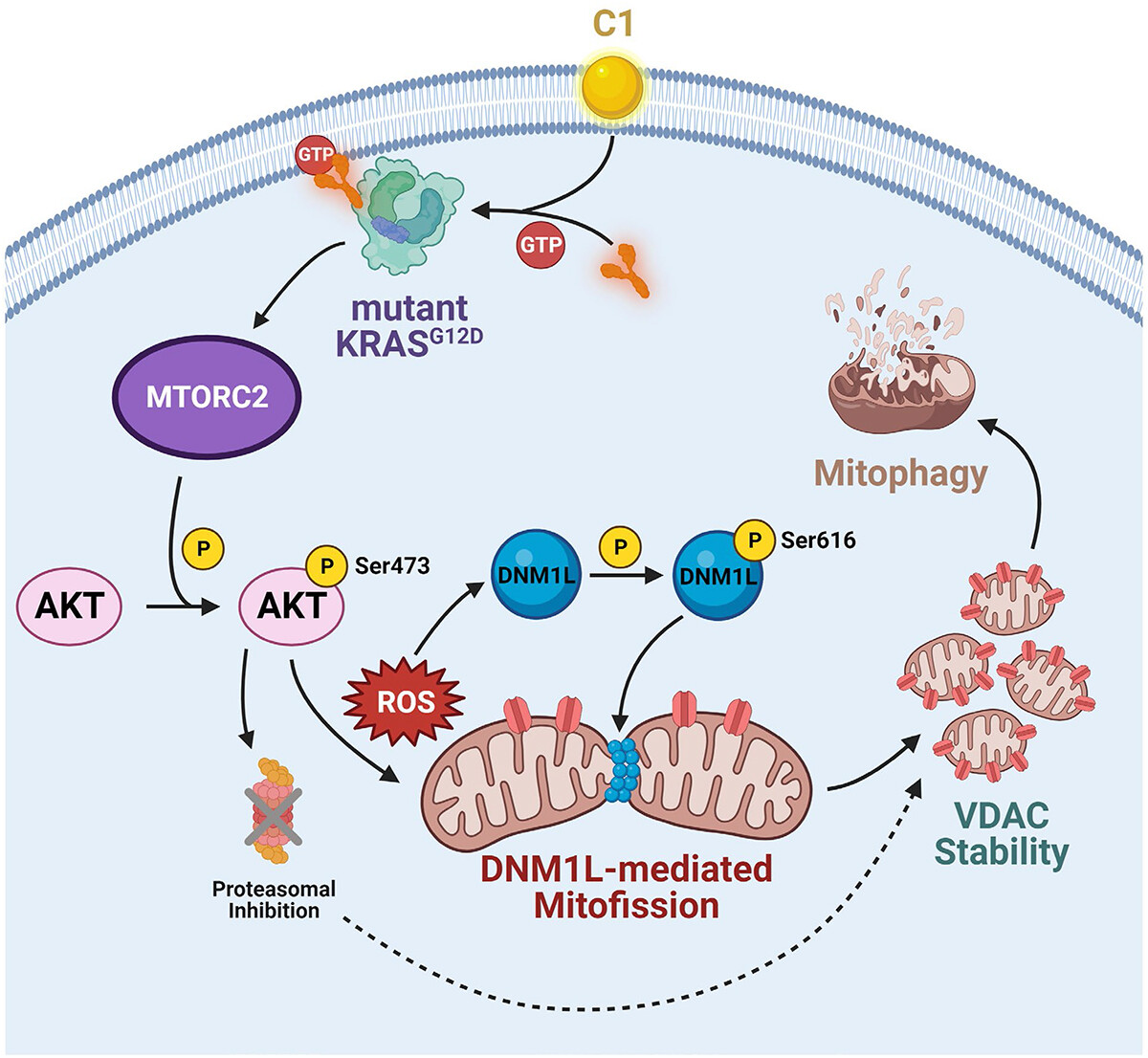
Oncogenic mutations of KRAS are associated with a host of human malignancies, in particular, pancreatic, colorectal and lung cancer. Therefore, there is heightened interest in the design and development of KRAS-specific inhibitors, however, with modest clinical response. A recent report (Iskandar, K. et al. Autophagy, 2024) from the group of Professor Shazib Pervaiz at the Department of Physiology and N2CR, YLL SoM, has unravelled a novel molecular mechanism for the targeted execution of mutant KRAS driven tumors. This work specifically exploited the ability of mutant KRAS to trigger oxidative stress upon activation. “Defying conventional dogma, here we demonstrate the ability of a small molecule to selectively execute mutant KRAS expressing cancer cells via hyperactivation rather than inhibition of KRAS activity”, remarked Kartini B. Iskandar, the first author on this publication.
Investigating the underlying mechanism of the small molecule revealed mutant KRAS-driven activation of the kinase AKT that triggered oxidative stress and fragmentation of the cell’s powerhouse, the mitochondria. Notably, MTOR complex 2 (MTORC2) is identified as a critical player in this execution signal as inhibition of MTORC2 alleviated oxidative stress and restored the ability of cancer cells to migrate, invade and form spheroids. These findings provide a novel insight into the potential design of therapeutic strategies to exploit cancer cell’s dependence on mutant KRAS activation induced signaling. “For decades attempts at directly targeting KRAS have been modestly successful, hence obviating the need for identifying novel signaling nodes for selective drug design. Our work provides a proof of concept by leveraging on cancer’s Achilles heel and making cells drown in their addiction”, commented Professor Pervaiz.
Read the paper here: https://www.tandfonline.com/doi/full/10.1080/15548627.2024.2307224#