
My research area is based on earlier work whereby during development of the neural crest when NGF becomes limiting, neuronal progenitors that are out-competed for NGF turn on a specific apoptotic program through EglN3 (Lee et al., 2005; Straub et al., 2003). EglN3 is an oxygen sensor and a component of the oxygen-sensing pathway that was awarded The Nobel Prize in Physiology or Medicine in 2019. Indeed, this form of developmental apoptosis is oxygen-dependent and important for determining the final number of terminally differentiated cells, failure of which may lead to excessive progenitor cells, thus increasing the likelihood of accumulating subsequent mutational hits and accelerating tumor development (Sommer and Rao, 2002; Zhu and Parada, 2002). Indeed, studies have demonstrated links between escape from NGF withdrawal culling and pediatric nervous system cancers including neuroblastoma and medulloblastoma (Katsetos et al., 2003).
Given the importance of this EglN3-dependent culling process, our previous work sought to investigate the precise mechanism downstream of EglN3. Through an unbiased genome-wide shRNA library loss-of-function screen, we identified kinesin KIF1Bβ as necessary and sufficient to induce neuronal apoptosis when NGF becomes limiting (Schlisio et al., 2008). More intriguingly, KIF1Bβ maps to 1p36.22, a chromosomal region that is frequently deleted in neural crest-derived tumors including neuroblastoma, pheochromocytoma and melanoma. Sequencing a large set of neuroblastoma, pheochromocytoma and medulloblastoma cases, we identified several loss-of-function missense mutations in KIF1Bβ, arguing that KIF1Bβ could indeed be a pathogenic target of these deletions, thus allowing progenitors to escape culling and setting the stage for tumor initiation (Schlisio et al., 2008). We then went on to address the mechanism of KIF1Bβ-induced apoptosis in neuroblastoma and during developmental NGF withdrawal.
We identified a transcriptional basis for KIF1Bβ-induced apoptosis, which requires a RNA/DNA helicase known as RNA helicase A (DHX9). KIF1Bβ interacts with DHX9 to promote translocation of cytoplasmic DHX9 into the nucleus, resulting in transcription of apoptotic XIAP-associated factor 1 (XAF1)(Chen et al., 2014). Transcription-impaired or nuclear localization-impaired DHX9 is unable to potentiate KIF1Bβ-induced cell death. Knockdown of DHX9 also protects from KIF1Bβ-induced cell death whereas KIF1Bβ negative mutants are unable to translocate cytoplasmic DHX9 into the nucleus. Furthermore, XAF1 silencing protects from KIF1Bβ-induced apoptosis (Chen et al., 2014). Recent literature strongly pointed to KIF1Bβ as a bonafide tumor suppressor.
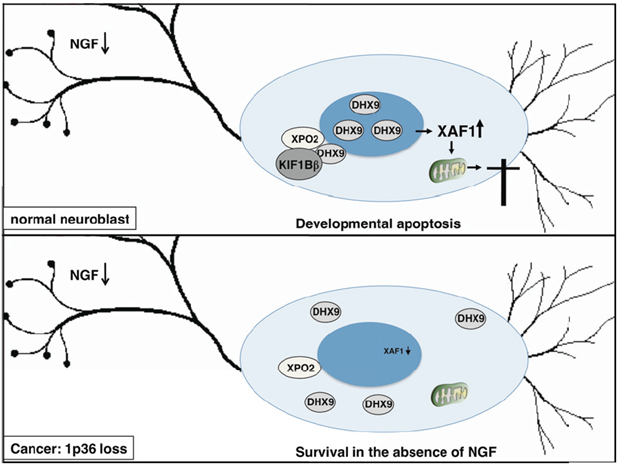
Our findings provide a mechanistic understanding of this role, whereby KIF1Bβ interacts with cytoplasmic DHX9 leading to its accumulation in the nucleus to initiate a unique transcriptional signature that includes apoptotic effectors such as XAF1 (Chen et al., 2014)(Fig 1).
Through the developmental biology and relevant perturbations that we uncovered to be responsible for the development of neuroblastoma, particularly the reported inhibition of XIAP by XAF1, we postulated XIAP as a potential novel druggable target that could be exploited for high risk, difficult-to-treat and relapsed-prone neuroblastomas (Liston et al., 2001; Bernards, R., 2014)(Fig 2). To study this, we collaborated with Curis, Debiopharm, Genentech, Novartis and University of Haifa. Through in vitro experiments using commercial and patient-derived cell lines, we identified a first-in-class compound ‘A4’, an ARTS mimetic, as most efficacious and least toxic. However, A4 is the only current XIAP specific antagonist that effectively kills neuroblastoma cells. The potential of other drugs that might work better than A4 in terms of efficacy in targeting XIAP and killing neuroblastoma has yet to be fully explored. We are currently amidst a high through screening to investigate other potential drugs with similar XIAP specific degradation mechanism of action which can be used as a novel treatment option for neuroblastoma beyond the current small molecule A4. Furthermore, we are expanding the XIAP degradation pathway with A4 and other promising drugs, as a therapeutic strategy in adult cancers such as ovarian and colorectal cancers. We aim to investigate the XIAP degradation pathway with A4 and potential drugs as a therapeutic strategy outside of pediatric cancer, in adult cancers.
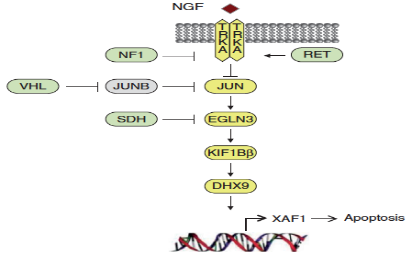
Fig 2: Inhibition of XIAP as a consequence of XAF1-mediated apoptosis during NGF withdrawal
Current methods of monitoring disease and evaluating minimal residual disease (MRD) in neuroblastoma by RT-qPCR and immunocytology of urinary and bone marrow markers provide limited quantitative and biological information, and is invasive in the case of obtaining bone marrow aspirate (BMA). To overcome this, using a label-free cell size-based separation method, we enriched and characterized intact circulating cells in peripheral blood indicative of neuroblastoma CTCs, as well as their DTC counterparts in the bone marrow. Expression profiles of pro-metastatic genes in CTCs correlated with the presence of bone marrow metastases at diagnosis, while longitudinal profiling identified persistently elevated expression of genes in CTCs that may serve as novel predictive markers of hematogenous MRD in neuroblastoma patients that subsequently relapse (Loh et al., 2022).
Prognosis for advanced-stage, metastatic and relapsed neuroblastomas remains poor in Asia compared to North America and Europe. Furthermore, the profiles of neuroblastoma genes such as those on 1p36 are not well characterized in Asian patients. Hence, we sought to identify novel variants and evaluate the prevalence/penetrance of known 1p36 genetic variants that may render their loss in expression vis-à-vis loss-of-function. In a recently published study, we profiled the mutational variants in our neuroblastoma patients by evaluating pairs of tumor specimens and adjacent normal tissue with a custom targeted sequencing panel covering neuroblastoma candidate genes located at 1p and at other chromosomal loci. Among the variants identified, BRCA2 was noted to be one of the most frequent (Kuick et al., 2022). Functional assays are currently underway to further characterize these variants and understand the role of BRCA2 in neuroblastoma and its effect on treatment response, particularly DNA damaging agents.
Impact of XIAP inhibition and its mechanisms on neuroblastoma suppression
Drug discovery for treatment of refractory neuroblastoma based on XIAP biology
Mutational and functional analysis of cancer predisposition genes in neuroblastoma
Impact of BRCA2 inhibition and its mechanisms on neuroblastoma treatment with DNA damaging cytotoxics