Anthony Khong
Affiliations
- Assistant Professor, Department of Physiology, National University of Singapore
- NUS Centre for Cancer Research Translational Research Programme, Translational Research Programme
Research Areas of Interest:
The Khong lab aspires to describe how biomolecular condensates participate in cancer biology with the goal of developing cancer therapeutics.
Biochemical processes are often carried out at subcellular locations in eukaryotic cells. One approach that eukaryotic cells use to compartmentalize biochemistry is through lipid membranes. Alternatively, eukaryotic cells can also compartmentalize biochemistry without using lipid membranes through the self-assembly of proteins and nucleic acids. These structures are known as biomolecular condensates. Some examples of biomolecular condensates include the nucleolus, paraspeckles, nuclear speckles, P-bodies, and stress granules (Figure 1).
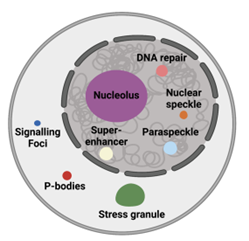
Figure 1: Examples of biomolecular condensates found in eukaryotic cells
Biomolecular condensates are involved in a range of biological processes including DNA replication and repair, telomere maintenance, transcription, RNA metabolism and trafficking, misfolded protein quality control, stress resistance, and signal transduction. Cancers and tumors have usurped biomolecular condensate biology to promote tumorigenesis.
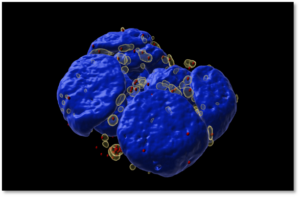
One particular biomolecular condensate that my laboratory is interested in is stress granules. These are transient cytosolic biomolecular condensates that form during acute stress, hence the name “stress granules” (Figure 2). Stressors that can induce stress granules include oxidative stress, hypoxia, and nutrient deprivation. Stress granules assemble from the coalescence of accumulated non-translating messenger ribonucleoproteins during cellular stress.
Figure 2: Stress granules induced by oxidative stress in mouse embryonic stem cell colony.
Stress granules (transparent yellow), individual mRNAs that accumulate in stress granules (red), and nuclei (blue).
Stress granules are important in cancer biology because they are thought to participate in a range of cancer processes including transformation, stress resistance, and metastasis. But the molecular mechanisms that explain how stress granules are involved in tumorigenesis remain opaque.
My laboratory is interested in elucidating how stress granules are involved in tumorigenesis. Moreover, we plan to map out the protein-protein interactions facilitating granule formation in order to develop novel anti-stress granule therapeutics. Furthermore, in collaboration with the NUS Centre for Cancer Research (N2CR) in Singapore, we plan to survey when and where stress granules are prevalent in cancers and tumors. The overall goal is to advance stress granule biology in cancer research.