The Cancer Initiation and Evolution Laboratory (CIEL) is focused on understanding key events which occur along the continuum of normalcy to cancer. This includes the first changes which sets a cell on the path towards eventual cancer, as well as later events which may dictate metastasis or confer onto a cancer unique oncologic phenotypes. Currently, the laboratory has a special interest in colorectal cancer.
Principal Investigator Dedrick Chan is concurrently appointed Consultant Colorectal Surgeon at the Division of Colorectal Surgery at the National University Hospital, as well as Junior Academic Fellow with the Department of Surgery, National University of Singapore. He also holds an honorary posion as Principle Investigator with the Institute of Molecular and Cell Biology, Agency for Science Technology and Research. Atier completion of surgical training, Dedrick underwent doctoral training at the University of Oxford with a fully-funded scholarship by the National Medical Research Council. There, he conducted leading research using advanced molecular biology techniques involving organoids as well as gene editing. This culminated in his research being awarded a prize at the 10th Annual CRUK Oxford Centre Symposium, a coming together of cancer research conducted within Oxford across all disciplines. Dedrick’s interests are in characterizing the role of driver mutations in normal colonic tissue, particularly in relation to how these could either accelerate or inhibit cancer initiation. Dedrick is also interested in developing new techniques involving patient-derived human organoids, as well as novel gene editing strategies. Focus
Focus Areas
The lab is interested in the molecular changes which occur on the continuum from normalcy, to pre-cancer, and finally to cancer. Cancers themselves are highly heterogeneous, and individual patient’s cancer characteristics are likely to be traceable to unique molecular perturbations.
To achieve this, we use patient-derived organoid models coupled with CRISPR-Cas9 gene editing technology.
Currently, we have ongoing projects with the following interests
a. Characterising the role of driver mutations in normal colonic tissue
b. Uncovering the mechanisms of increased EMT and lymph node metastasis in colon cancer
c. Molecular features of peritoneal metastasis
A gene edited patient-derived organoid with knockout of the APC gene. Scale bar, 100μm.
a. Dr Sriram Sridharan, Cancer Institute of Singapore, NUS – Determining whether mutational signatures at microsatellite repeats can be used as biomarkers for treatment in MSI
colorectal cancer
b. Dr Liang Kaicheng, Institute of Molecular and Cell Biology, ASTAR, and Dr Gwyneth Soon, Department of Pathology, NUH – Using deep ultraviolet-excited fluorescence histology to provide real-time tissue architecture to aid in diagnosis of colorectal polyps
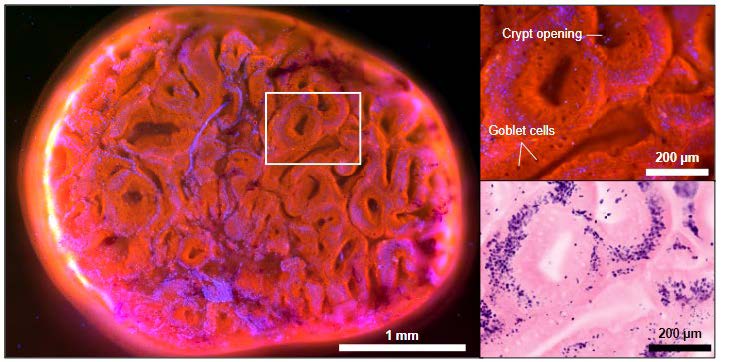
Polyp stitched image (leti), single field-of-view (FOV) and recolored image to simulate H&E appearance (right), 10X. Pathologist reported as tubular adenoma, low-grade dysplasia. Acquisition time: 2minfor 3 x 3 mm sample

