Issue 44
Nov 2022
ETHICALLY SPEAKING
By Dr Victor Cole, Senior Research Fellow, Centre for Biomedical Ethics (CBmE), NUS Medicine

The COVID-19 pandemic brought about an urgent examination of the practicalities of conducting clinical research in the midst of a public health emergency. In particular, the use of newly emergent technologies such as Zoom videoconferencing to facilitate remote interaction between researchers and research participants drew much attention.
While moving at speed to adopt such methods of working remotely, the taking of consent and the gathering of data from research sites has necessitated urgent ethical deliberation. The possibility of retaining the use of such methods beyond the exigencies of events such as pandemics has also come very much to the fore, not least because of the cost savings and efficiencies they may bring about.
The possibility of retaining certain remote research methods after the primary reason for adopting them has retreated motivated a half-day online workshop on the ethics of remote consent taking and remote data gathering, conducted by staff from the NUS Centre for Biomedical Ethics (CBmE) in July this year. This event was run under the ‘Science, Health and Policy-relevant Ethics in Singapore’ (SHAPES) initiative hosted by CBmE and supported by the Ministry of Health’s National Medical Research Council. It attracted 99 participants, primarily from the research and ethical oversight fields.
Invited speakers included Ms Sumitra Sachidanandan, Regulatory Consultant from the Health Sciences Authority, and biomedical researchers, Dr Austin Ang Chin Siang from the Centre for Population Health Sciences, Nanyang Technological University, and Dr Aaron Tan from the National Cancer Centre Singapore. Panel discussions involving these technical specialists and ethicists from CBmE preceded detailed case study discussions. In this article, I outline some of the issues that the workshop participants grappled with.
Remote consent-taking
In the context of a pandemic situation, a key ethical consideration in moving to a remote consent-taking model is the minimisation of the risk of infection to study participants and research team members. Beyond general risk at the population level, there may also be increased levels of risk and thus vulnerability among individual sufferers of particular medical conditions, such as Multiple Sclerosis, should they be exposed to infectious diseases such as COVID-19. This further motivates consideration of remote consent-taking methods. This is a consideration based upon the principle of beneficence.
However, any risks associated with in-person consent need to be weighed against other risks associated with remote consent taking. In order for a potential research participant to exercise their autonomy in reasonably assessing the potential benefits and drawbacks of participating, there has to be sufficient possibility of interaction with the consent taker for them to be able to seek clarification and understand the nature of the risks they might be exposed to. This may be considered particularly pertinent in higher risk studies, though there is no clear line that can be drawn between studies which require in-person consent and those that do not on the basis of a perceived level of risk inherent to the study itself.
“In order for a potential research participant to exercise their autonomy in reasonably assessing the potential benefits and drawbacks of participating, there has to be sufficient possibility of interaction with the consent taker for them to be able to seek clarification and understand the nature of the risks they might be exposed to.”
It should also be noted that there is no certainty that remote consent-taking would result in a reduction of the potential for interaction, given that some individuals may feel more at ease sharing their concerns and seeking additional information when physically distanced from the consent taker. There are thus nuances of study design and study population that need to be attended to in making risk-based judgements affecting the choice of consent-taking method.
A further motivation to act beneficently toward research participants in adopting remote consent-taking approaches might derive more simply from the reduction of inconvenience; for example, in relation to the need to travel to the research site in order to provide consent.
Making further provision for the potential research participant to make a choice between consenting remotely or in person may be seen as most fully honouring the principle of autonomy, though this would have to be considered in light not only of safety risks but also of additional costs that making provision for both would likely impose.
Another concern arising from using video recording in consent-taking relates to potential violations of privacy, both through data breach where unauthorised parties might gain access to recordings, and through capturing images of non-consenting parties who just happen to be in the background of the images recorded. This is particularly possible in a home setting.
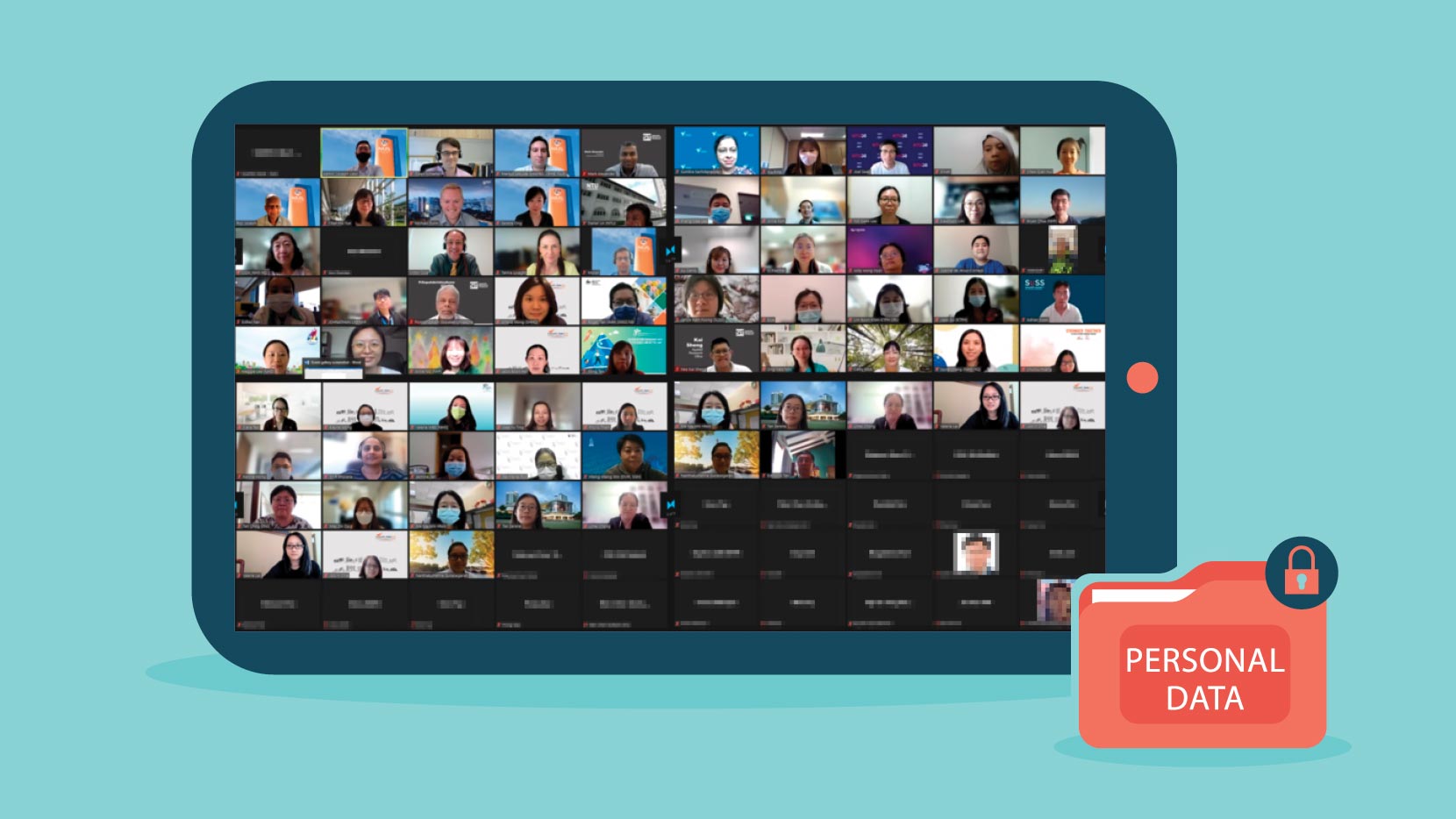
As important as the need to avoid capturing unwitting parties in video recordings is the need to ensure that the consent given is witnessed by an impartial party. The Health Sciences Authority’s Guidance on Electronic Consent1 requires the presence of such a witness for all new trial participants if the e-consent is taken remotely.
A justice consideration arises in deciding whether to move to remote consent-taking where potential participants might be excluded by virtue of a lack of access to the technological means by which the consent is obtained, or by having insufficient technological competence in managing such means where they are available.
Additionally, those with visual or auditory impairments might be disadvantaged, depending on the extent to which the remote consent-taking requires the use of those faculties.
Conversely, the use of remote consent-taking tools might make it easier for some disadvantaged groups to participate in the research than would otherwise have been the case; for example, those with physical impairments that restrict their mobility.
“In making the decision of whether or not to adopt a decentralised model for clinical trials, the minimisation of burdens to study participants (including the reduction of risks) has to be weighed against the downsides, which may include impacts on the quality of data generated and thus the social value of the study outcomes.”
Other considerations in remote consent-taking, such as the ability of the consent taker to verify the identity of the consent giver have ethical significance for the integrity of the study and its ultimate potential for producing public benefit, though these are essentially practical concerns rather than ethical ones relating to whether consent should be obtained remotely as opposed to in-person.
Additional benefits to remote consent-taking include its potential for enhancing audit and documentation processes, though these would not be reasons by themselves to adopt such an approach. Ultimately, the decision to move to remote consent-taking is a multifactorial one in which the interests of the would-be study participants would be paramount.
Remote data gathering
Many of the ethical concerns that arise in relation to remote consent-taking appear again when considering whether to adopt remote data gathering methods. Having information conveyed electronically to the study team by research participants themselves increases the risk of data breaches, and a loss of privacy should those communications be intercepted by hackers or otherwise misappropriated.
Whether activities that research participants may be engaged in need to be recorded in the first place also needs to be considered. If the study outcomes are not dependent on a video recording being available for analysis, then there is no clear case for recording despite the benefits that might arise from enhancing the potential for audit and review. Once again, the inadvertent capture of unwitting persons in the background of recordings may be considered an additional privacy risk.
Study participants’ self-submission of data via remote means has the potential for undermining data reliability and thus the social value the research is ultimately intended to produce. Data which participants find particularly sensitive, such as weight, may be more likely to be misreported than those that have little or no bearing on self-image.

Again, the specifics of the study need to be considered in terms of what the risks to data quality are. The arrangements for safety monitoring are important considerations in any clinical trial, though serious adverse events would typically be self-reported anyway and would not be expected to align with scheduled study visits.
Payments to study participants to compensate them for expenses and inconvenience incurred would very likely need to be reduced for remote studies compared to those conducted on site, given the lack of need for travel. However, changing to remote monitoring mid-trial because of an intervening factor such as the COVID-19 pandemic creates some complication for how compensation is reasonably calculated.
In making the decision of whether or not to adopt a decentralised model for clinical trials, the minimisation of burdens to study participants (including the reduction of risks) has to be weighed against the downsides, which may include impacts on the quality of data generated and thus the social value of the study outcomes. Fundamentally, of course, no study should proceed on a remote basis if doing so has predictably deleterious consequences for the well-being of the participants. With that said, a variety of benefits can accrue from the adoption of a decentralised model in terms of recruitment, representation, cost and convenience, such that close attention to the various ethical considerations raised above is merited.
The author wishes to acknowledge Assistant Professor Owen Schaefer and Mr Markus Labude, who developed the workshop materials on which this article is based.
A further iteration of SHAPES’ online workshop “New Research Practices in the Wake of COVID-19: Remote Consent and Remote Data Gathering” will be offered in March or April 2023.
Reference document: https://www.hsa.gov.sg/docs/default-source/hprg-io-ctb/hsa_ctb_guidance_e-consent_6nov2020.pdf.
More from this issue