Cellular Immunotherapy: Harnessing the Body’s Defenses against Cancer
In this second installment of the Cancer Immunotherapy series, we delve into cellular immunotherapy, which involves harnessing the power of immune cells to attack cancer cells.
First, let’s meet the lead characters: the natural killer (NK) cell and the T cell, which are two of the immune system’s most effective killers. NK cells are part of the body’s first line of defense, specialised in detecting intruders (foreign organisms or cells). Interestingly, they can differentiate between cancer cells and normal cells. However, NK cells are relatively short lived, surviving for about a week in the body. T cells, on the other hand, can multiply in large numbers and remain in the body for months or even years. When NK or T cells recognise cancer cells, they are triggered to release toxic substances that kill the cancer cells.
AGAINST BLOOD CANCERS
At present, cellular immunotherapy is mainly used in clinical trials. NK and T cells can be taken from a patient, expanded (multiplied), and infused back into the same patient. In other cases, NK or T cells are taken from a donor, expanded, and infused into a patient. This may require matching of cell proteins to prevent rejection of the donor cells. Donor NK cells have shown promising results in blood cancers such as acute myeloid leukaemia.1,2
Another intensive area of research involves modifying special proteins on T cells called receptors such that the T cells now recognise and kill cancer cells effectively. Each type of modified T cell recognises a specific type of cancer. The modified cells, called chimeric antigen receptor T (CAR-T) cells, are expanded to obtain the large numbers (ranging from tens of millions to hundreds of billions) generally needed for therapy. CAR-T cells have shown impressive results in patients with leukaemia and lymphoma. In three trials involving 65 patients with acute lymphocytic leukaemia, 70% to 90% of patients showed a complete response to the treatment.3-5
AS WELL AS SOLID TUMOURS
Besides leukaemia and lymphoma, researchers are beginning to test cellular immunotherapy as treatment for solid tumours such as melanoma as well as breast, colorectal, liver, head and neck, and non-small-cell lung cancers. At the National University Cancer Institute, Singapore (NCIS), Associate Professor Lee Soo Chin is studying the effect of activated NK cells and trastuzumab (Herceptin®), an antibody that acts against a protein called HER2, in 22 patients with metastatic HER2-positive breast cancer and gastric cancer.
Trastuzumab mainly works by blocking the proliferation of cancer cells that are triggered by HER2. The antibody also harnesses the patient’s own NK cells to fight against the cancer, a process called antibody-dependent cell-mediated cytotoxicity or ADCC (see Figure). This mechanism is usually only a small part of trastuzumab’s action because of the small numbers and weak activity of NK cells in most cancer patients.
However, in A/Prof Lee’s study, each patient’s NK cells are expanded an average of 300 times and activated in a special culture in Professor Dario Campana’s laboratory, then infused back into the same patient in combination with trastuzumab. In this way, the ADCC process is enhanced for better cancer control. Importantly, the NK cells from these patients with advanced cancer could be expanded to the same magnitude as cells from healthy volunteers. Also, the expanded cells are fully functional, showing high ability to kill cancer cells in the laboratory.
In the 11 patients who have received the treatment (trastuzumab and at least 10 million NK cells per kilogram of body weight), the disease stabilised in most of them, extending the time until their cancer worsened to more than eight months. This is encouraging as these patients had already been unsuccessfully treated with an average of six different types of therapy for metastatic cancer, including trastuzumab (one patient had tried 13 different treatments before this trial). Their cancers would almost certainly have progressed without effective treatment. The trial is ongoing and thus far, the therapy appears to be safe, with only relatively mild and transient side effects in two patients. Next, A/Prof Lee intends to test CAR-T cells, which are more effective killers than NK cells, in such patients.
Another NUS Medicine researcher, Dr Lim Chwee Ming from the Department of Otolaryngology, is evaluating the effectiveness of NK cells in head and neck cancers, which are especially prevalent in South and Southeast Asia.6 One type, nasopharyngeal cancer (NPC), is linked to infection from the Epstein-Barr virus. Dr Lim has started using activated NK cells and an antibody, cetuximab (Erbitux®), to treat patients with metastatic NPC and head and neck squamous cell carcinoma who have failed several other treatments. Similar to A/Prof Lee’s observations, the NK cell therapy appears to be halting progression of tumours in the two patients who have been treated to date. As more patients are treated, Dr Lim will evaluate this cell-antibody combination as a potential treatment avenue for NPC patients with very limited options.
A GAME CHANGER IN CANCER TREATMENT
Cellular immunotherapy has been revolutionary for blood cancers such as leukaemia and lymphoma, and shows promise against several types of solid tumours. As researchers test this therapy in more patients and in those with less advanced cancer, a clearer picture is beginning to form about this powerful weapon in the fight against cancer. Weighing the benefits on one hand, and cost and side effects on the other hand, will be an increasingly important consideration for clinicians.
Since many factors are at play in the tumour micro-environment, some cancers will probably need a multi-faceted approach. For example, different combinations of NK or T cell therapy, checkpoint inhibitors,* cancer gene inhibitors, and chemotherapy are being explored to find the best offensive line against various cancers.
*Antibodies that release cancer’s chokehold on the immune system; see the first article in this series.
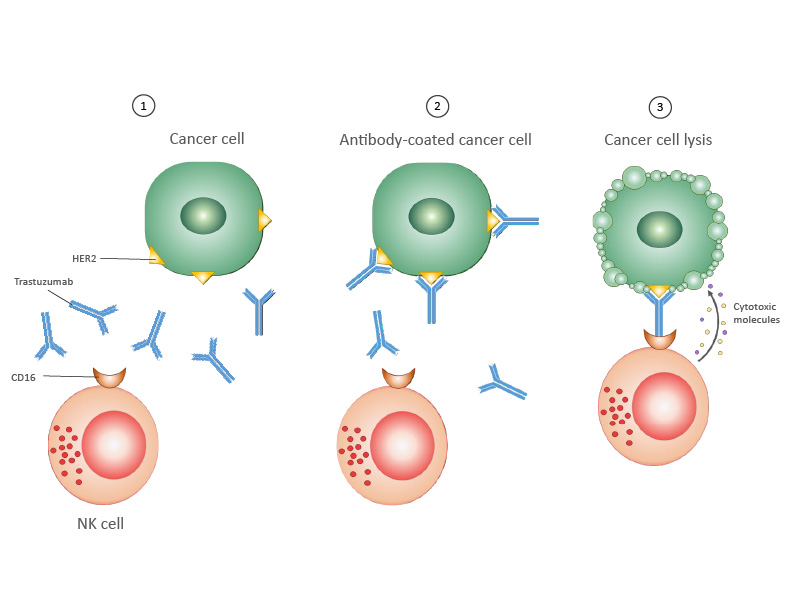
1. Breast cancer cell expressing HER2 protein on its surface. An antibody targeting HER2, trastuzumab, is given to the patient.
2. Trastuzumab binds to HER2 on the cancer cell.
3. The NK cell binds to trastuzumab. Once bound, the NK cell releases cytotoxic molecules, which kill the cancer cell.
Credits: Dr Koh Shimin Grace, Department of Paediatrics, NUS
• NK cells carry a mix of inhibitory and activating receptorson cell surface
• At any time, net result of inhibitory and activating signals shifts balance to either “ignore” or “engage”
• NK cells use this balance to differentiate between normal cells and cancer cells
• Differs from T cell activation, which requires binding of specific ligands to T-cell receptor and co-receptor
References
1. Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955-959.
2. Bachanova V, Cooley S, Defor TE, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123:3855-3863.
3. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507-1517.
4. Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25.
5. Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528.
6. Head and Neck Tumors. Cancer Network. http://www.cancernetwork.com/cancer-management/head-and-neck-tumors. Published June 1, 2016. Accessed March 8, 2017
