Insights from multi-omic modeling of neurodegeneration in xeroderma pigmentosum using an induced pluripotent stem cell system
Insights from multi-omic modeling of neurodegeneration in xeroderma pigmentosum using an induced pluripotent stem cell system
Cherif Badja, Sophie Momen, Gene Ching Chiek Koh, Soraya Boushaki, Theodoros I. Roumeliotis, Zuza Kozik, Ian Jones, Vicky Bousgouni, Jo~ao M.L. Dias, Marios G. Krokidis, Jamie Young, Hongwei Chen, Ming Yang, France Docquier, Yasin Memari, Lorea Valcarcel-Zimenez, Komal Gupta, Li Ren Kong, Heather Fawcett, Florian Robert, Salome Zhao, Andrea Degasperi, Yogesh Kumar, Helen Davies, Rebecca Harris, Christian Frezza, Chryssostomos Chatgilialoglu, Robert Sarkany, Alan Lehmann, Chris Bakal, Jyoti Choudhary, Hiva Fassihi, and Serena Nik-Zainal
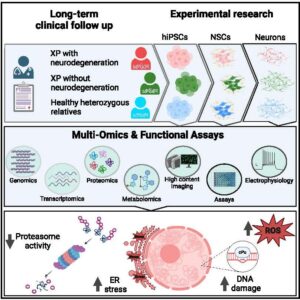
Xeroderma pigmentosum(XP) is caused by defective nucleotide excision repair of DNA damage. This results in hypersensitivity to ultraviolet light and increased skin cancer risk, as sunlight-induced photoproducts remain unrepaired. However, many XP patients also display early-onset neurodegeneration, which leads to premature death. The mechanism of neurodegeneration is unknown. Here, we investigate XP neurodegeneration using pluripotent stemcellsderivedfromXPpatients andhealthy relatives, performingfunctional multi-omicsonsamples duringneuronaldifferentiation.We showsubstantially increasedlevels of 50,8-cyclopurineand8-oxopurine in XPneuronalDNAsecondary tomarked oxidative stress. Furthermore,we find that the endoplasmic reticulum stress response is upregulated and reversal of the mutant genotype is associatedwith phenotypic rescue. Critically, XP neurons exhibit inappropriate downregulation of the protein clearance ubiquitin-proteasome system (UPS). Chemical enhancement of UPS activity in XP neuronal models improves phenotypes, albeit inadequately. Although more work is required, this study presents insights with intervention potential.
Full article: https://www.cell.com/cell-reports/fulltext/S2211-1247(24)00571-0