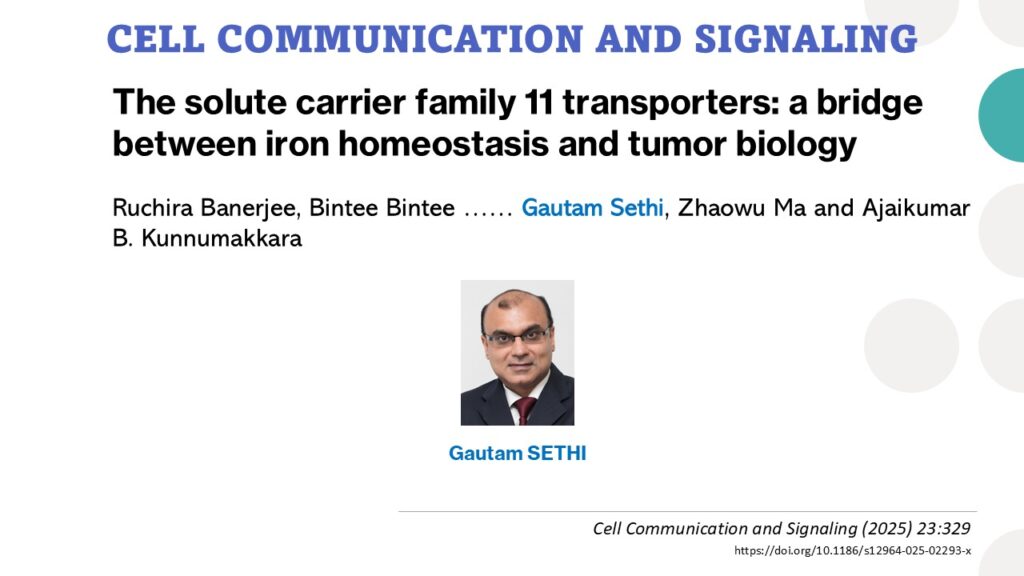
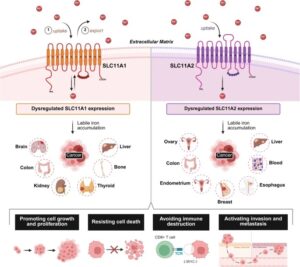
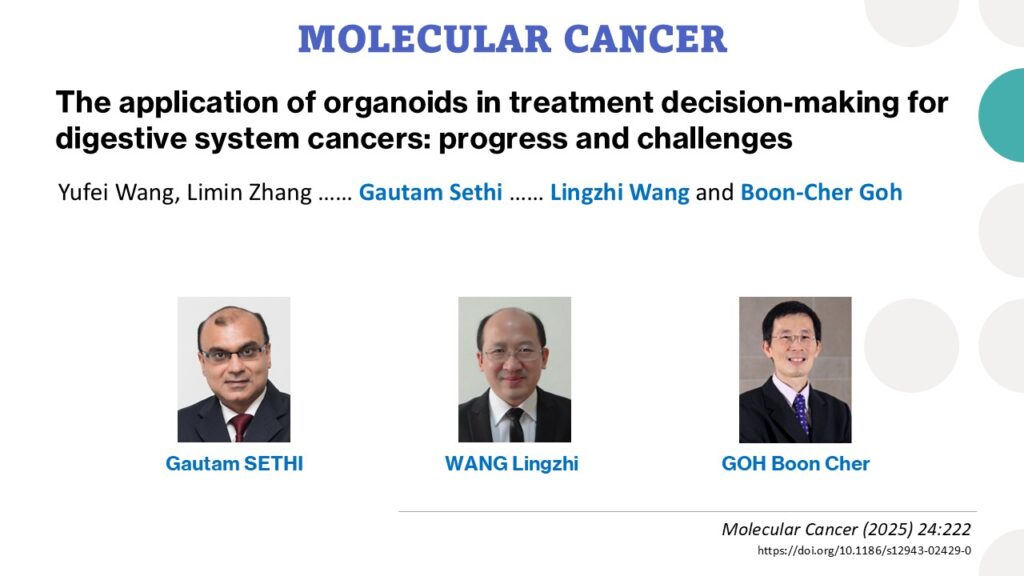
Extracellular Vesicles Administered via Intrathecal Injection Mediate Safe Delivery of Nucleic Acids to the Central Nervous System for Gene Therapy

ABSTRACT
Gene therapy holds great potential for treating neurological disorders, but its implementation is limited by the challenge of developing a safe and effective delivery method to the central nervous system (CNS). Red blood cell-derived extracellular vesicles (RBCEVs) have the potential to address these challenges due to their non-immunogenicity, non-cytotoxicity, ability to be redosed, and suitability for nucleic acid loading. In this study, we demonstrate the efficacy and safety of RBCEV-mediated nucleic acid delivery to the CNS. We found that RBCEVs administered through intrathecal injection are widely distributed across the CNS and efficiently taken up by neuronal cells. Delivery of RBCEVs loaded with GFP-encoding plasmids results in GFP expression in neurons. Our data also highlight the potential of RBCEVs to deliver plasmids encoding secretory proteins, resulting in protein secretion within the cerebrospinal fluid. Furthermore, experiments conducted in both mouse and non-human primate models indicate that intrathecal injection of plasmid-loaded RBCEVs do not lead to any systemic or local acute toxicity. In summary, our findings illustrate the potential of the RBCEV-based platform as a viable and safe approach for nucleic acid delivery to the CNS, facilitating further development of gene therapy for neurological disorders.
Full Article: https://isevjournals.onlinelibrary.wiley.com/doi/10.1002/jev2.70116