
Issue 51
Aug 2024
SCIENCE OF LIFE
The Institute of Mental Health (IMH), in collaboration with the Yong Loo Lin School of Medicine, National University of Singapore (NUS Medicine), has embarked on two clinical pilots to study the efficacy of personalised Transcranial Magnetic Stimulation (TMS) in treating persons with treatment-resistant depression (TRD).
The trials, named APIC-TMS (Asia Pacific Individual Connectomics – Transcranial Magnetic Stimulation) and SPARK-D (Singapore’s Precision Approach for Relief from Depression), are catalysed by Temasek Foundation (TF) and the National Medical Research Council (NMRC) respectively, with a grant of S$1 million each.
Dr Tor Phern Chern, Senior Consultant, Department of Mood & Anxiety and Head of Neurostimulation Service, IMH, is the Principal Investigator for both clinical trials.
Major depressive disorder and TRD
Major Depressive Disorder, commonly known as “depression”, is the most common psychiatric disorder among adults in Singapore. The 2016 Singapore Mental Health Study found that 6.3% of the Singapore adult population—or 1 in 16 adults (aged 18 and above)—has experienced the disorder at some point in their lifetime.
Mild cases of depression may be managed with psychotherapy, while moderate to severe cases are typically managed with antidepressant medication to alleviate the symptoms. Yet, some patients may not reach the remission stage despite taking medications and undergoing therapies. Clinically, this is known as TRD.
There are no local studies on the prevalence of TRD. Figures from overseas literature vary with ranges between 12% to 55%1 and 40% to 70%2, depending on how the studies define TRD and the study methodologies.
TRD is usually managed through a stepwise treatment approach that includes optimising medication dosage, switching to a different class of medication, and augmenting or combining treatments, with the inclusion of non-pharmacological treatments such as electroconvulsive therapy (ECT) and standard TMS.
Standard TMS versus Personalised TMS
Standard TMS delivers stimulation to the same spot of the brain for all participants, using a manually manipulated holder.
On the other hand, personalised TMS is more targeted and precise. It uses individualised functional magnetic resonance imaging (fMRI) to find the ideal location in each patient’s brain that should be stimulated to treat his/her depression, and a high precision robot arm to target the stimulation.
This spot in the brain is unique for each individual and to identify it, an algorithm developed by Research Fellow Ruby Kong and Associate Professor Thomas Yeo from the Centre for Sleep and Cognition at NUS Medicine and NUS College of Design and Engineering, will be used to perform advanced analytics on the individual brain scans.
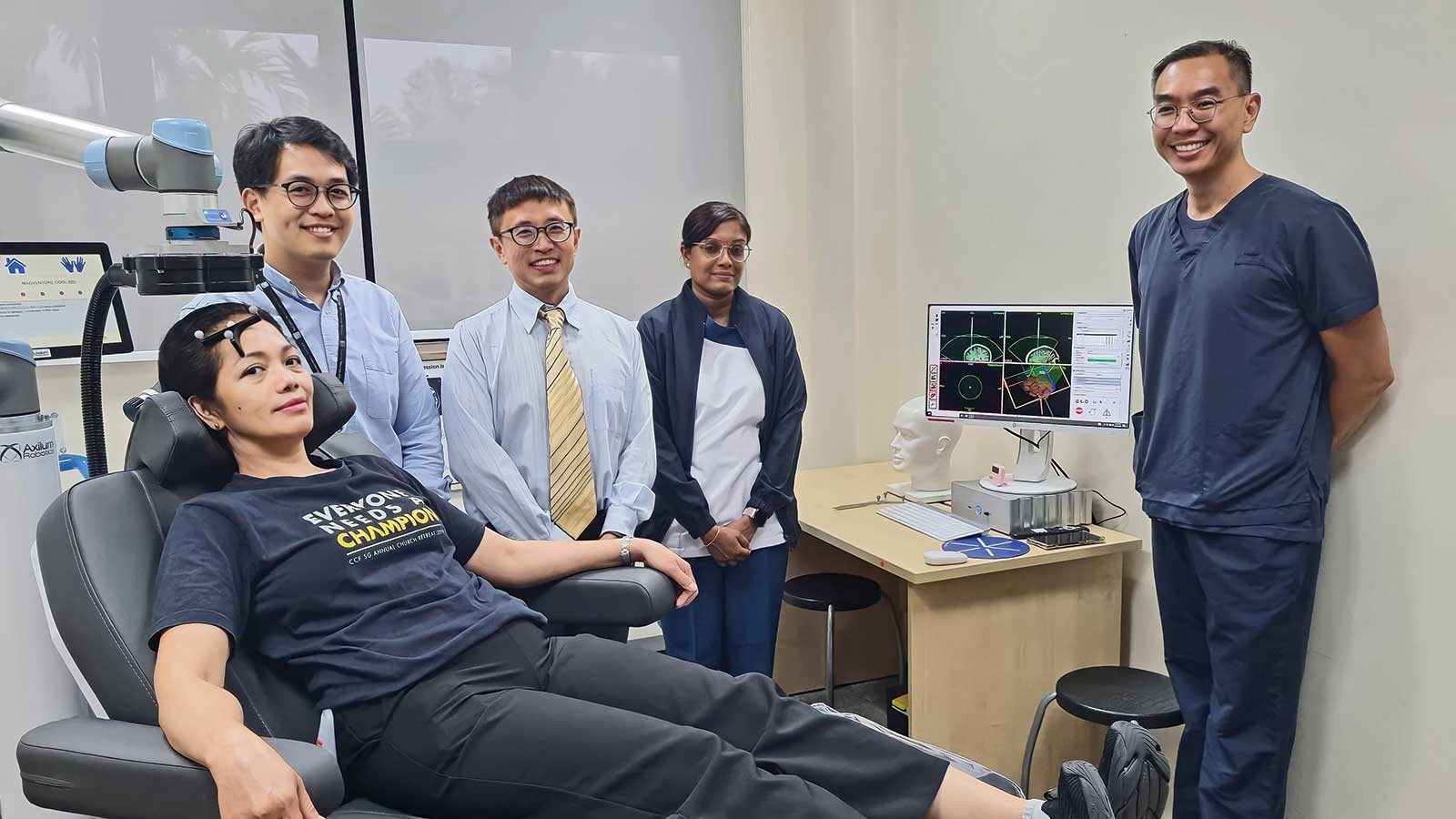
From left to right: Nurse Loida G Sawit, Dr Jonathan Lee, Consultant in the Department of Mood & Anxiety at the Institute of Mental Health (IMH), Associate Professor Thomas Yeo, Deputy Director of the Centre for Translational Magnetic Resonance at NUS Medicine, Nurse Geeta Kasinathan and Dr Tor Phern Chern, Senior Consultant in the Department of Mood & Anxiety and Head of Neurostimulation Service at IMH demonstrating how personalised TMS is applied on patients with TRD.
A/Prof Thomas Yeo, who is also Deputy Director of the Centre for Translational Magnetic Resonance Research at NUS Medicine and co-PI for the clinical trials, said, “Functional MRI is one of the few non-invasive approaches that can safely image the living human brain. By using this newly-developed machine-learning algorithm to clearly outline high-quality individualised brain networks from the limited quantity of functional MRI data, we are able to locate the exact spot unique to each patient, and stimulate it to treat their depression disorder as accurately as possible. These upcoming pilot trials provide a platform to demonstrate that the safety and efficacy from personalised TMS can be assured for patients with TRD who are undergoing this procedure.”
By using this newly-developed machine-learning algorithm to clearly outline high-quality individualised brain networks from the limited quantity of functional MRI data, we are able to locate the exact spot unique to each patient, and stimulate it to treat their depression disorder as accurately as possible.”
80%
of patients achieved remission in personalised TMS pilots done with American subjects
The data from the fMRI scans is then integrated with an advanced neuro-navigation robotic arm to mount the magnetic coil on the precise spot on the patient’s head. Even if the patient moves during treatment, the robotic arm will keep the coil firmly trained on the spot so that stimulation can be precisely delivered without disruption to that patient.
Singapore is the first country in Southeast Asia to conduct such clinical trials of personalised TMS, modelled after the Stanford Accelerated Intelligent Neuromodulation Therapy (SAINT) protocol. In pilots done with American subjects who had TRD, approximately 80% of patients achieved remission with SAINT. The Singapore pilots will pair IMH’s clinical expertise in standard TMS with NUS Medicine’s expertise in brain MRI to personalise a treatment plan for each participant, study its efficacy and make recommendations on implementing this as a mainstream treatment.
Currently, ECT is the gold standard treatment for TRD, with a remission rate of 50% to 60%. In comparison, the SAINT pilot has shown that remission rates improve significantly to about 80% with personalised TMS after 1 week.
A cohort of 20 participants with TRD will be recruited for the APIC-TMS trial, and 70 for the SPARK-D trial. Participants will have to undergo fMRI scans as well as TMS. APIC-TMS will offer participants the personalised target, and SPARK-D will randomly assign patients to either the personalised target or the current standard one-size-fits all target. Both clinical trials are set to run concurrently from March 2024 to 2026.
The Prevalence and National Burden of Treatment-Resistant Depression and Major Depressive Disorder in the United States | Psychiatrist.com.
Questions and Answers about the NIMH Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Study — Background - National Institute of Mental Health (NIMH) (nih.gov).
More from this issue



