New Hope for Paediatric Leukaemia Patients
In recent years, advances in chemotherapy and anti-cancer antibodies have improved the cure rates for several leukaemias and lymphomas. However, the prognosis for patients who fail to respond to chemotherapy or whose cancer recurs after an initial response remain poor. One of the biggest challenges for these patients is the lack of effective treatment options.
This bleak landscape was brightened with the discovery of an innovative therapy, CAR-T cell therapy, which was due in large part to the work of Professor Dario Campana, the Mrs Lee Kong Chian Chair in Advanced Cellular Therapy and Director of the Division of Immunopathology and Cell Therapy at the Department of Paediatrics, NUS Medicine. In recognition of his contributions, Prof Campana was conferred the 2019 Jacob and Louise Gabbay Award, along with Professor Michel Sadelain, Director of the Center for Cell Engineering at the Memorial Sloan Kettering Cancer Center. In October, Prof Campana travelled to Brandeis University in Massachussetts, USA, to receive the award and give a presentation about his work.2
The CAR-T cell therapy involves taking a patient’s T cells (the immune system’s attack machines) and modifying their receptors to chimeric antigen receptors (CAR), which recognise the cancer cells. This produces CAR-T cells that can hunt down and kill the cancer cells.3
CAR-T cell therapy has shown impressive results in patients with leukaemia and lymphoma. In four trials involving a total of 140 patients with relapsed acute lymphocytic leukaemia (ALL), 70% to 90% of patients showed complete remission in the first few months after CAR-T cell treatment and up to 80% of patients maintained complete remission for 1 year after treatment.1-4 This is in contrast to the 17% of relapsed ALL patients who achieved complete remission with chemotherapy.5
Based on these and other encouraging results, two CAR-T cell therapies have now been approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Axicabtagene ciloleucel (Yescarta®, Gilead) was approved by the FDA in 2017 and the EMA in 2018 for the treatment of relapsed or refractory diffuse large B-cell lymphoma.6,7 Tisagenlecleucel (Kymriah®, Novartis) was approved by the FDA in 2017 for the treatment of relapsed or refractory B-cell ALL in patients up to 25 years of age,8 and for relapsed or refractory diffuse large B-cell lymphoma in 2018.9 It was approved by the EMA for treating both cancers in 2018.10
Although CAR-T cell therapy is not yet available on the Singapore market, some patients are receiving it through clinical trials. For example, Prof Campana and Associate Professor Allen Yeoh, Viva-Goh Foundation Associate Professor in Paediatric Oncology and Head, Division of Paediatric Haematology and Oncology, NUS Medicine, have treated 10 patients with high-risk ALL with CAR-T cell therapy. These patients, who range in age from 3 to 28 years, had not responded well to chemotherapy, with a significant number of cancer cells remaining after treatment. Thus far, the 10 patients in the trial are responding well to the CAR-T cell therapy.
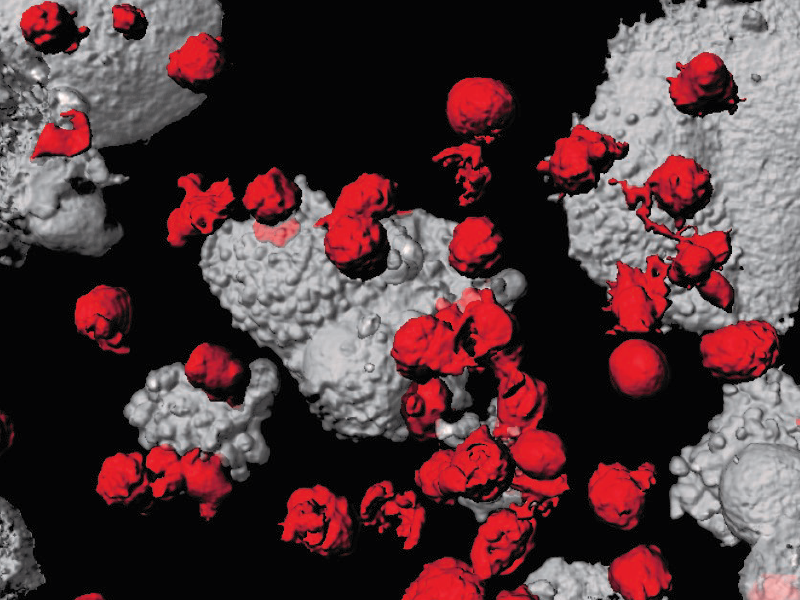
CAR-T cells (red) attacking cancer cells (grey)
Profs Campana and Yeoh are now also testing the CAR-T cell therapy in adults with ALL, who have poorer outcomes with chemotherapy than children affected by ALL. If results are promising, this immunotherapy could provide much-needed hope for a group of patients who have very limited treatment options.
Towards the goal of making more cell therapies available to patients, Prof Campana has started three companies, including MediSix in Singapore as well as Unum Therapeutics and Nkarta Therapeutics, both in the U.S., to commercialise therapies for leukaemias, lymphomas and other cancers.
“CAR-T cell therapy has revolutionised the treatment of patients with ALL, who are resistant to chemotherapy,” said Prof Campana. “Our vision is that cellular therapy will become a standard component of the treatment for leukaemia, lymphoma, and other forms of cancer.”
References
1. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507-1517.
2. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439-448.
3. Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25.
4. Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517-528.
5. von Stackelberg A, Volzke E, Kuhl JS, et al. Outcome of children and adolescents with relapsed acute lymphoblastic leukaemia and non-response to salvage protocol therapy: a retrospective analysis of the ALL-REZ BFM Study Group. Eur J Cancer. 2011;47:90-97.
6. U.S. Food and Drug Administration. FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma. October 18, 2017.
https://www.fda.gov/newsevents/ press-announcements/fda-approves-car-t-celltherapy- treat-adults-certain-types-large-b-cell-lymphoma.
Accessed September 3, 2019.
7. Gilead Sciences, Inc. Yescarta® (Axicabtagene Ciloleucel) Receives European Marketing Authorization for the Treatment of Relapsed or Refractory DLBCL and PMBCL, After Two or More Lines of Systemic Therapy. August 27, 2018.
https://www.gilead.com/news-and-press/press-room/ press-releases/2018/8/yescarta-axicabtagene-ciloleucelreceives- european-marketing-authorization-for-thetreatment- of-relapsed-or-refractory-dlbcl-and-pmbcl-aftertwo- o.
Accessed September 3, 2019.
8. U.S. Food and Drug Administration. FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome. September 7, 2017.
https://www. fda.gov/drugs/resources-information-approved-drugs/ fda-approves-tisagenlecleucel-b-cell-all-and-tocilizumabcytokine- release-syndrome.
Accessed September 3, 2019.
9. U.S. Food and Drug Administration. FDA approves tisagenlecleucel for adults with relapsed or refractory large B-cell lymphoma. May 3, 2018.
https://www.fda.gov/drugs/ resources-information-approved-drugs/fda-approvestisagenlecleucel- adults-relapsed-or-refractory-large-b-celllymphoma..
Accessed September 3, 2019.
10. Novartis AG. Novartis receives European Commission approval of its CAR-T cell therapy, Kymriah® (tisagenlecleucel). August 27, 2018.
https://www.novartis. com/news/media-releases/novartis-receives-europeancommission- approval-its-car-t-cell-therapy-kymriahtisagenlecleucel..
Accessed September 3, 2019.

Prof Dario Campana (standing) with Assoc Prof Allen Yeoh (second from left) and their patients.
