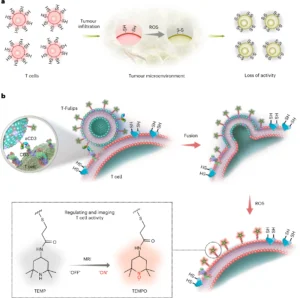
T cells play a determining role in the immunomodulation and prognostic evaluation of cancer treatments relying on immune activation. While specific biomarkers determine the population and distribution of T cells in tumours, the in situ activity of T cells is less studied. Here we designed T-cell-targeting fusogenic liposomes to regulate and quantify the activity of T cells by exploiting their surface redox status as a chemical target. The T-cell-targeting fusogenic liposomes equipped with 2,2,6,6-tetramethylpiperidine (TEMP) groups neutralize reactive oxygen species protecting T cells from oxidation-induced loss of activity. Meanwhile, the production of paramagnetic 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO) radicals allows magnetic resonance imaging quantification of the T cell activity. In multiple mouse models, the T-cell-targeting fusogenic liposomes led to efficient tumour inhibition and to early prediction of radiotherapy outcomes. This study uses a chemical targeting strategy to measure the in situ activity of T cells for cancer theranostics and may provide further understanding on engineering T cells for cancer treatment.