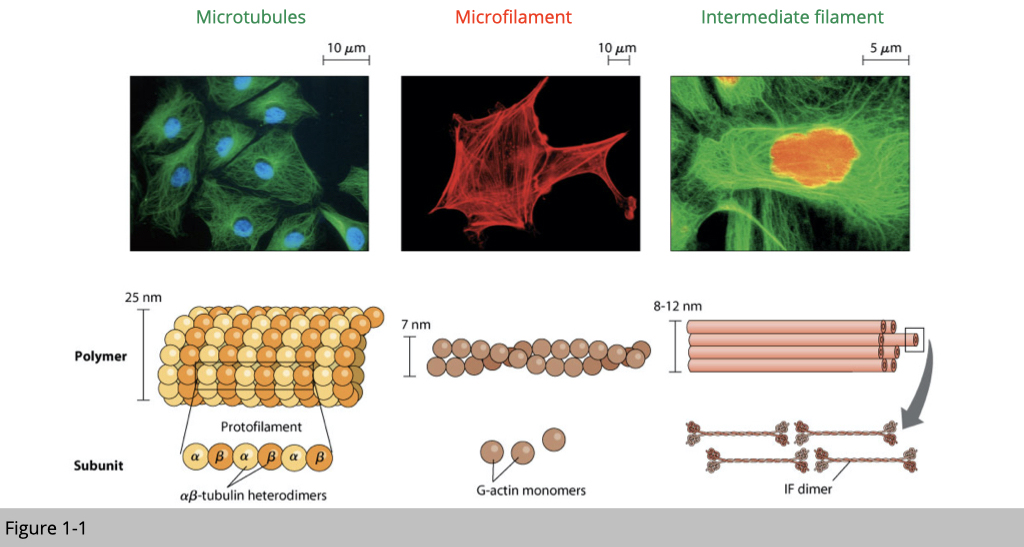
From the Figure 1-1, you can see that the diameter of microtubules is 3.5 times bigger than microfilament and 2-3 times bigger than intermediate filament. From the staining, you also notice that the distribution of microtubules (labeled in green) mainly reside in the cytoplasm, microfilaments (labeled in red) mostly locate on the surface of a cell, and intermediate filaments (labeled in green) are like tension-bearing elements, which hold up cell shape and serve to anchor in place several organelles and cell itself to the extracellular matrix.
Neurons have elaborate cytoskeletal structures.
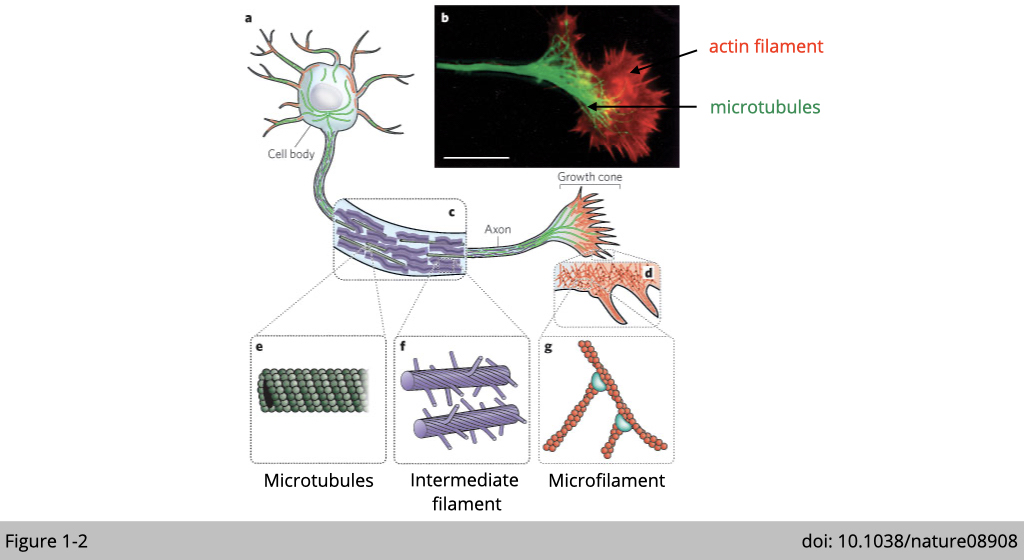
Why the location is matter? The location of a protein within the cell often suggests something about its function. For example, a protein such as an insulin receptor that recruits binding of insulin from serum is located at the plasma membrane. Figure 1-2 is a simplified diagram of a neuron cell. Each mammalian neuron consists of a cell body, dendrites, and an axon. The nucleus located in the cell body and dendrites extends from the neuron cell body to receive messages from other neurons. The axon also extends from the cell body and often gives rise to many smaller branches before ending at a synapse.
As you can see in the Figure 1-2, the microtubules labeled in green, originated from the cell body along the axon to the synapse. This long microtubule is perfect for axon transportation, in which the newly synthesized biomaterial is transported out from cell bodies to supply distal axons. The damaged or old proteins and organelles are transported back from axon to cell bodies to be degraded. It is not surprised that abnormalities of the microtubule systems of axons and dendrites contributing to many neurodegenerative diseases.
Another type of cytoskeletal protein, actin filament, on the other hand, is enriched in presynaptic axonal terminals and postsynaptic dendrites. Actin filaments play an important role for “vesicle trafficking”, which coordinate communication between cells. Dysfunctions in actin dynamics have significant consequences for cognition and are closely associated with neurological disorders and dementia.
Neurofilaments (NF) labeled in purple in the diagram are classed as type IV intermediate filaments found in the cytoplasm of neurons. Neurofilaments provide structural support for the establishment of axon. When neurons or axons degenerate, neurofilament proteins are released into the blood or cerebrospinal fluid. Therefore, neurofilament is a useful marker for monitoring neurodegenerative disease such as Huntington's disease.
Immunofluorescence.
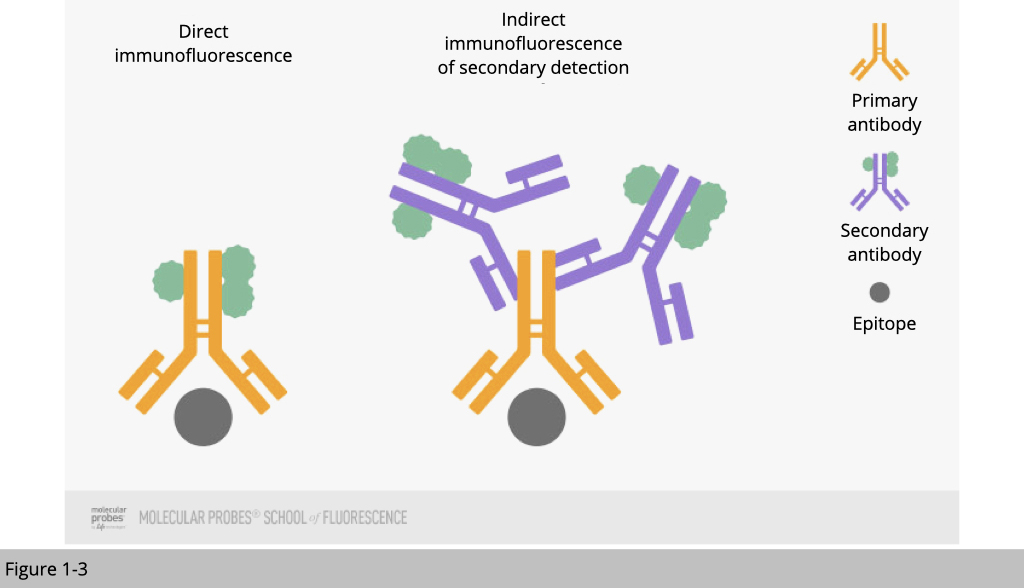
The most common way to detect location of a protein is to apply immunofluorescence(IF) staining. Using fluorophore-tagged antibodies to label the protein of interest, we detect the cellular location of the target protein by observing the fluorophore signal. There are two IF methods depending on whether the fluorophore is conjugated to the primary or the secondary antibody:
- Direct IF uses a single antibody against the target of interest. The primary antibody is directly conjugated to a fluorophore.
- Indirect IF uses two antibodies. The primary antibody is unconjugated and a fluorophore-conjugated secondary antibody directed against the primary antibody is used for detection.
Fluorescent-protein tagging.
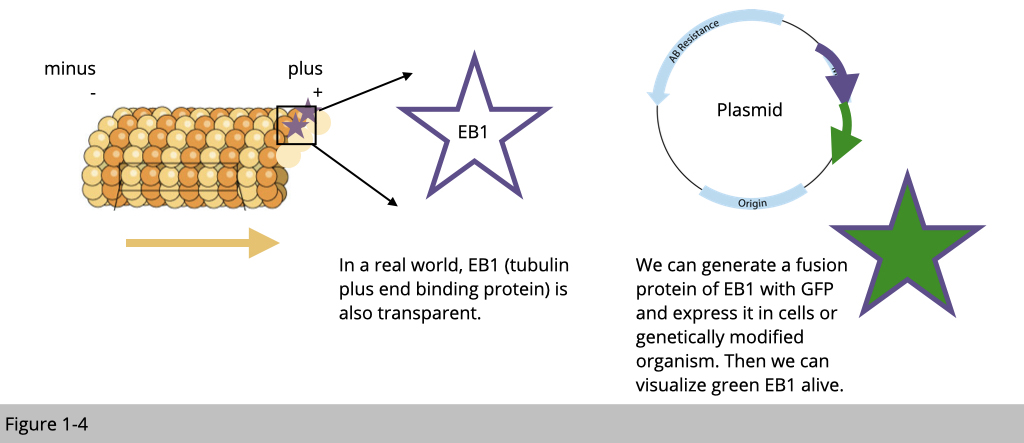
2008 Nobel prize went to Osamu Shimomura, Martin Chalfie and Roger Y. Tsien "for the discovery and development of the green fluorescent protein, GFP.” Why it is a cutting-edge discovery? It is because now we can make a protein of interest visible by fusing it with a fluorescent protein. In this case, EB1 proteins bind to microtubule plus ends so they accumulate at growing microtubule ends. When EB1 fused with GFP, we can follow the green signal to detect the direction and estimate the speed of microtubule growth in a living cell.
Fluorescent dyes.
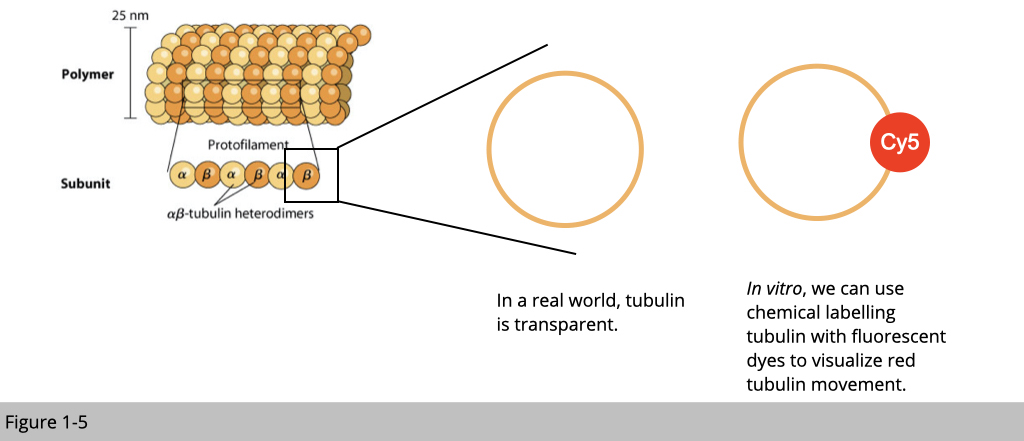
We can also use chemical reaction to covalently link the fluorescent dye to the surface of tubulin in vitro. In this case, the tubulin is red under microscope.
Limitation of light microscope.
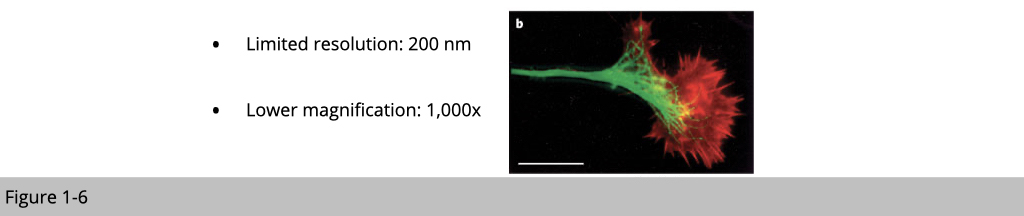
Yet, there is a limitation of light microscope. The resolution of light microscope is 200 nm. The actual power or magnification of a compound optical microscope is the product of the powers of the ocular (eyepiece) and the objective lens. The maximum normal magnifications of the ocular and objective are 10× and 100× respectively, giving a final magnification of 1,000×. For example, mitochondria is typically ranging in size from 0.5 µm to 1 µm in length. If we want to study whether a protein X located in outer or inner membrane of mitochondria, the best light microscope in the world can’t not differentiate them. We need something else.
Electron microscope.
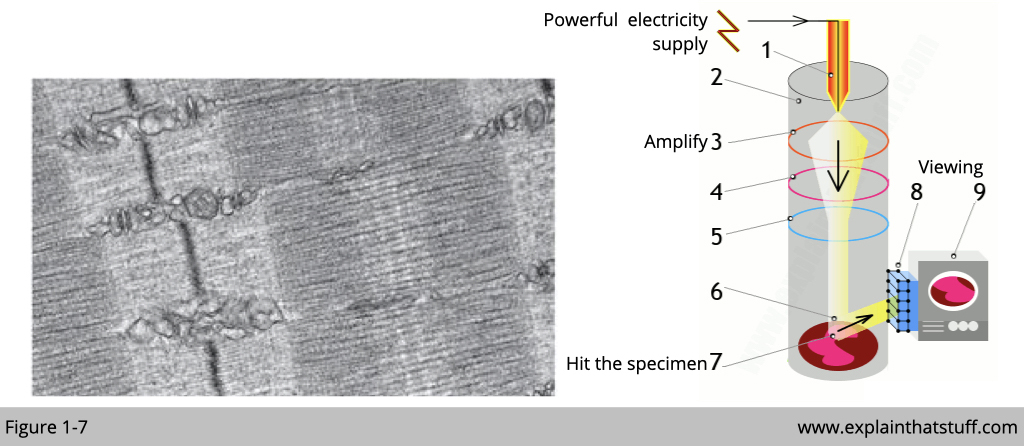
Electron microscope is invented to overcome the limitation from light microscope and this is another Nobel prize awarded to Ernst Ruska, Gerd Binnig and Heinrich Rohrer in 1986. Similar to light which is a photon in physic, electrons have a shorter wavelength than visible light, and this allows electron microscopes to produce higher-resolution images than standard light microscopes. A scanning transmission electron microscope has achieved better than 50 pm resolution in regular dark-field imaging mode and magnifications of up to about 10,000,000×. Another type of EM- cryoEM even can be used to study a single protein to a resolution of approximately 1.2 ångströms.
More readings: https://www.nature.com/articles/d41586-020-02924-y