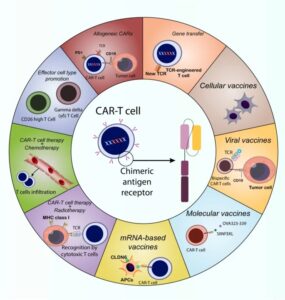
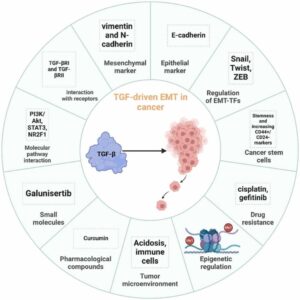
Unlocking therapeutic potential: Exploring nuclear receptors in brain cancer treatment

Abstract
Brain cancer remains among the most lethal malignancies worldwide, with approximately 321,476 new cases and 248,305 deaths reported globally in 2022. The treatment of malignant brain tumors presents substantial clinical challenges, primarily due to their resistance to standard therapeutic approaches. Despite decades of intensive research, effective treatment strategies for brain cancer are still lacking. Nuclear receptors (NRs), a superfamily of ligand-activated transcription factors, regulate a broad range of physiological processes including metabolism, immunity, stress response, reproduction, and cellular differentiation. Increasing evidence highlights the involvement of NRs in oncogenesis, with several members demonstrating altered expression and function in brain tumors. Aberrations in NR signaling, encompassing receptors such as androgen receptors, estrogen receptors, estrogen-related receptors, glucocorticoid receptors, NR subfamily 4 group A, NR subfamily 1 group D member 2, NR subfamily 5 group A member 2, NR subfamily 2 group C member 2, liver X receptors, peroxisome-proliferator activated receptors, progesterone receptors, retinoic acid receptors, NR subfamily 2 group E member 1, thyroid hormone receptors, vitamin D receptors, and retinoid X receptors, have been implicated in promoting hallmark malignant phenotypes, including enhanced survival, proliferation, invasion, migration, metastasis, and resistance to therapy. This review aims to explore the roles of key NRs in brain cancer, with an emphasis on their prognostic significance, and to evaluate the therapeutic potential of targeting these receptors using selective agonists or antagonists.
Unlocking therapeutic potential: Exploring nuclear receptors in brain cancer treatment Read More »