Grant Period:
01 Mar 2020 – 28 Feb 2025 (Extended to Feb 2026)
Quantum:
S$847,250
Funding Source:
National Research Foundation and Singapore-ETH Centre (SEC)
Principal Investigator:
Tamra Lysaght / Owen Schaefer
Project Summary
This project is part of the Future Health Technologies (FHT) research programme within the Singapore-ETH Centre (SEC) (For more information on SEC, please go to: https://sec.ethz.ch/). This project (Module 4 of FHT) serves two primary purposes: (1) to develop and implement trustworthy data governance practices for FHT researchers engaged in Modules 1 through 3 and (2) to contribute high-quality scholarly work to the fields of bioethics and health law. To accomplish these objectives, Module 4 has initiated three value-creation projects focused on health data governance. These projects contribute to the development of a robust, trustworthy governance guidance, informed by ethical and legal considerations, that are of practical value to researchers working in health research and address critical gaps in knowledge of health data governance. The guidance will be particularly useful in helping stakeholders manage the intricacies of international data transfer while adhering to both legal requirements and ethical standards.
This proposal contains three projects:
|
Project |
Project themes |
|
1 |
Stakeholder engagement for trustworthy data governance |
|
2 |
Ethical decision making for the use of Artificial Intelligence in health care and research |
|
3 |
Wearable sensing technologies for health and research |
- Project 1 aims to develop trustworthy data governance framework through engagement activities with relevant stakeholders in Singapore to better reflect values that are important to them.
- Project 2 aims to collaboratively build evidence-based ethical decision-making support tools for the use of artificial intelligence (AI) in healthcare and research.
- Project 3 aims to explore the role of law in managing cross border data transfer in relation to wearable sensing technologies for health and research through conceptual legal analysis.
Project 1: Stakeholder engagement for trustworthy data governance
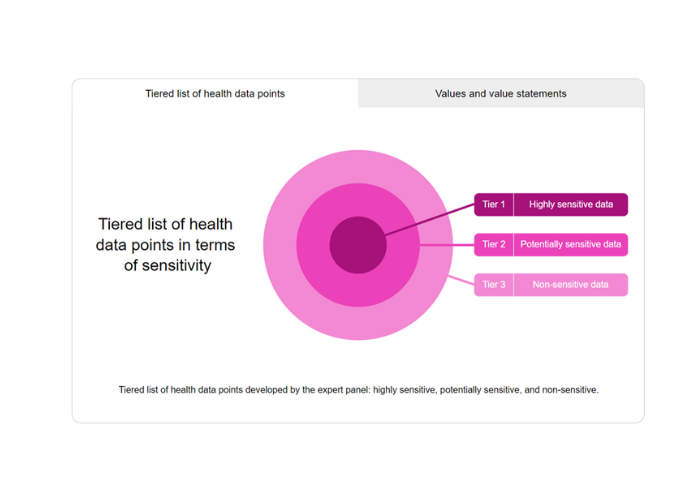
In 2022, the team conducted a modified Policy Delphi mixed-methods study to engage an expert panel of stakeholders in developing an ethical code that is both values-based and empirically informed on how researchers in Singapore should collect, use and transfer what constitutes potentially sensitive health data. The panel’s deliberations occurred over three stages starting with semi-structured interviews to generate ideas and policy options, which were then prioritised with an online survey and refined at a stakeholder workshop. Five stakeholder groups participated in the Policy Delphi: data contributors and end-users; data generators; data resources; data facilitators; and professional data users.
Please click on the following image to access the interactive diagrams.
Related Publications
• Lysaght, T., Chan, H.Y., Scheibner, J. et al. An ethical code for collecting, using and transferring sensitive health data: outcomes of a modified Policy Delphi process in Singapore. BMC Med Ethics 24, 78 (2023). https://doi.org/10.1186/s12910-023-00952-7
• Chan, H.Y., Toh, H.J. & Lysaght, T. Cross-jurisdictional Data Transfer in Health Research: Stakeholder Perceptions on the Role of Law. ABR (2024). https://doi.org/10.1007/s41649-024-00283-8