Research
(We are actively recruiting students and researchers at all levels. If interested in our research, please contact Dr Nguyen)
Our research: Understanding how small molecules are delivered to different cell types and organs.
The major research focuses in our lab are mechanistic characterizations of novel nutritionally regulated genes that involve in lipid transporters, nutritional transporters, and metabolism. We employ gene-knockout mouse models as well as various molecular cell biology, biochemical assays, and lipidomics, metabolite analysis approaches to mechanistically unravel the physiological and molecular functions of these novel proteins. The ultimate goals are to find molecular targets for therapeutic applications to treat diseases and improve health. We are actively pursuing the following themes:
1) Sphingosine-1-phosphate transport and signaling
Our lab recently cloned out a sphingosine-1-phosphate transporter, namely Mfsd2b (Vu et al., Nature 2017). Our current research is focused on the understanding of the roles of sphingosine-1-phosphate in cell signaling and functions. We have developed mouse models and cell-based to tackle this problem.
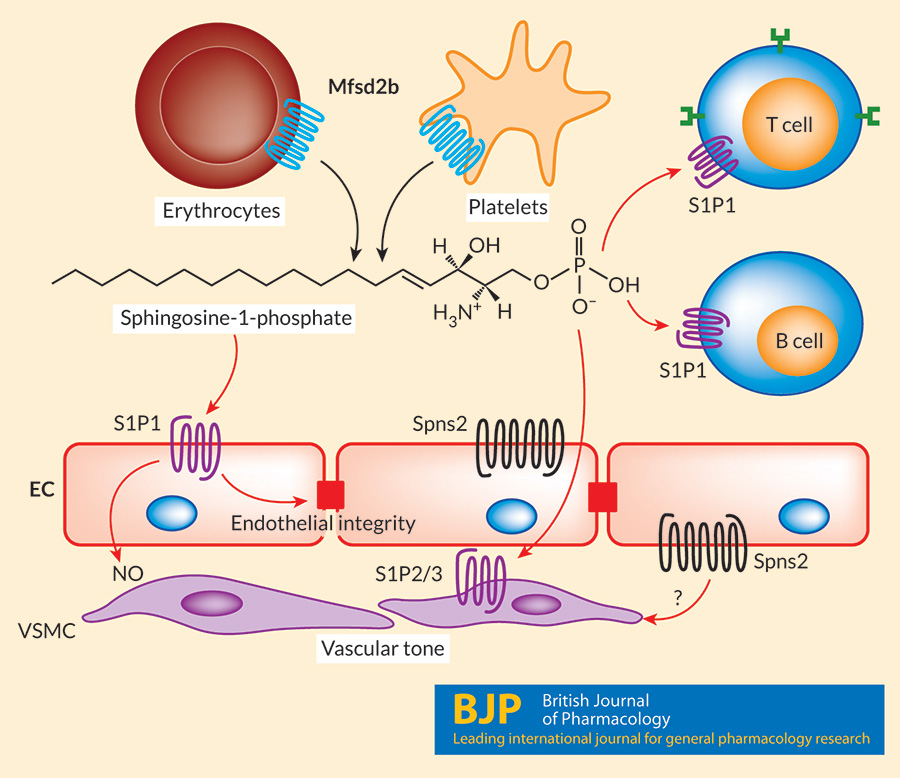
Sphingosine-1-phosphate is released out by Mfsd2b from hematopoietic cells and by Spns2 from endothelial cells. S1P plays roles as extracellular signaling lipid in which it activates 5 different G protein coupled receptors (GPCR) namely, S1PR1-5. S1PR1 is one of the most abundant receptor that is expressed in blood endothelial cells. This receptor is also expressed in T and B lymphocytes. The lipid also plays intracellular signaling roles. Much is remained to learn how S1P regulates pleiotropic cellular roles.
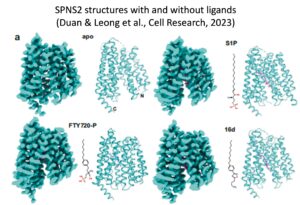
Solving atomic structures of S1P transporters. We worked with Dr He group from HIT, China to solve the first structures of human SPNS2. This work provides the molecular insights into the transport mechanism of S1P by SPNS2. Our study also lays the groundwork for understanding about how SPNS2 inhibitors compete with S1P to prevent its release from the cells.
2) Nutrient transporters at the blood-brain barrier
Our long standing interests are to characterize novel transporters at the blood brain barrier (BBB). Understanding the nutritional molecules that brain utilizes is vital for health and diseases. The uptake of nutrients for brain depends on mainly the active processes, which involve in protein transporters that are highly expressed in blood vessels in the brain. For example, the brain utilizes glucose as a primary source for energy production via glucose uptake by glucose transporter 1 (Glut1). The brain also utilizes fatty acids such as omega-3 fatty acids from fish, which are taken up via Mfsd2a (NLS1, sodium-dependent lysophosphatidylcholine symporter 1) in blood brain barrier. Much remains to be determined how brain takes up various nutrients, essential ions, vitamins, and many other molecules for its functions. Our lab has identified several transporters that are highly expressed in the BBB. For example, we recently report the roles of Mfsd7c for BBB development as well as for brain growth (Kalailingam, Wang, Toh, Nguyen,. et al. JCI 2020). We are actively working on the characterization of these proteins that we hope we will be able to shed lights on their roles as nutrition transporters for brain development and functions.
 |
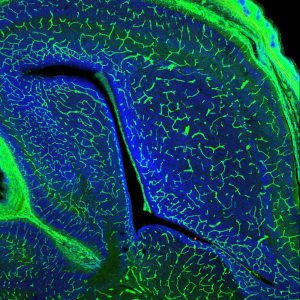 The blood-brain barrier in mouse brain (Green, blood vessels; Blue, nuclei) |
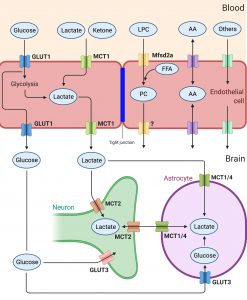
The role of solute transporters in regulation of nutrient uptake in the brain (GLUT, glucose transporter; MCT, monocarboxylic transporter; Mfsd2a, lysolipid transporter) |
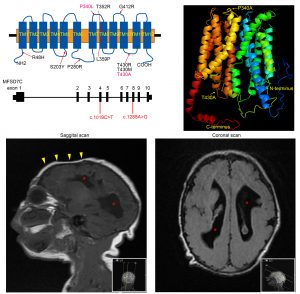 Human patients with Mfsd7c mutations (small brains) |
3) Lysosomal transport pathways for small molecules.
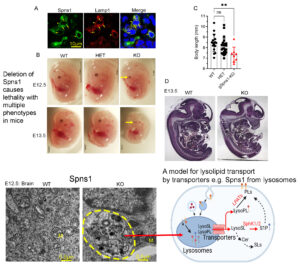
Lysosomes play a critical role in recycling macromolecules from cells by hydrolysis of these molecules to small ones and then release them via transporters. Defects in this process causes neumerous diseases such as Alzheimer’s; Parkinson; Lysosomal storage diseases. The lab is interested in identifying and characterizing the lysosomal membrane transporters. Recently, we identified Spns1 as a candidate for transporting lysolipids out of lysosomes. Deletion of Spns1 results in accumulation of these lipids in the lysosomes as such the animals with complete loss of Spns1 die before birth. Postnatal deletion of Spns1 in adult mice also causes phenotypes similar to lysosomal storage diseases. We are investigating the molecular and physiological roles of Spns1 and lysolipid transport from the lysosomes.