Development of a 3-dimensional printed tube thoracostomy task trainer: An improved methodology
Submitted: 2 April 2020
Accepted: 3 June 2020
Published online: 5 January, TAPS 2021, 6(1), 109-113
https://doi.org/10.29060/TAPS.2021-6-1/SC2243
Wen Hao Chen1, Shairah Radzi1, Li Qi Chiu2, Wai Yee Yeong3, Sreenivasulu Reddy Mogali1
1Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore; 2Department of Emergency Medicine, Tan Tock Seng Hospital, Singapore; 3Singapore Centre for 3D Printing, School of Mechanical and Aerospace Engineering, Nanyang Technological University, Singapore
Abstract
Introduction: Simulation-based training has become a popular tool for chest tube training, but existing training modalities face inherent limitations. Cadaveric and animal models are limited by access and cost, while commercial models are often too costly for widespread use. Hence, medical educators seek a new modality for simulation-based instruction. 3D printing has seen growing applications in medicine, owing to its advantages in recreating anatomical detail using readily available medical images.
Methods: Anonymised computer tomography data of a patient’s thorax was processed using modelling software to create a printable model. Compared to a previous study, 3D printing was applied extensively to this task trainer. A mixture of fused deposition modelling and material jetting technology allowed us to introduce superior haptics while keeping costs low. Given material limitations, the chest wall thickness was reduced to preserve the ease of incision and dissection.
Results: The complete thoracostomy task trainer costs approximately SGD$130 (or USD$97), which is significantly cheaper compared to the average commercial task trainer. It requires approximately 118 hours of print time. The complete task trainer simulates the consistencies of ribs, intercostal muscles and skin.
Conclusion: By utilising multiple 3D printing technologies, this paper aims to outline an improved methodology to produce a 3D printed chest tube simulator. An accurate evaluation can only be carried out after we improve on the anatomical fidelity of this prototype. A 3D printed task trainer has great potential to provide sustainable simulation-based education in the future.
Keywords: Medical Education, Chest Tube, Thoracostomy, Simulation, 3D Printing
I. INTRODUCTION
Training opportunities in procedures such as chest tube insertions are increasingly limited amidst a growing population of trainees. Yet, the deliberate practice remains essential to improving proficiency and preventing possible complications such as lung parenchymal damage (Hernandez, El Khatib, Prokop, Zielinski, & Aho, 2018). Hence, many institutions have adopted simulation-based training to provide realistic training opportunities while mitigating harm to patients.
Cadaveric and animal models are limited by access and cost, and raise religious and ethical concerns (Kovacs, Levitan, & Sandeski, 2018). In addition, commercial models tend to be very costly (e.g. Trauma-Man® at USD~$25,000). As such, new modalities are desired.
Three-dimensional (3D) printing can accurately recreate anatomical details from imaging data through precision modelling and a wide range of compatible printing materials (Mogali et al., 2018). Together with its decreasing cost, it has become an attractive technology for creating inexpensive and anatomically accurate simulation modalities.
A previous study from the Federal University of Parana, Brazil (Bettega et al., 2019) outlined the development and evaluation of a low-cost chest tube simulator. The bony structures were 3D printed, while the remainder of the model was manually assembled using silicone sheets, foam pads, and balloons.
They compared 2 groups of participants using a porcine rib model, and their 3D printed simulator respectively. They found subjective improvements in confidence and safety amongst both groups and showed no difference between the objective grades. Hence, they concluded that their 3D printed simulator was equivalent to the animal model concerning the simulation of a chest tube placement.
However, there exist many other 3D printing technologies and materials, which can potentially be applied to create superior haptics and anatomical detail. Hence, this paper aims to outline a methodology of integrating multiple 3D printing modalities to create a cost-efficient 3D printed chest tube simulator.
II. METHODS
An anonymised computerized tomography (CT) file of a healthy human thorax (2.5 mm slices thickness) in Digital Communication in Medicine (DICOM) format was downloaded from the databank provided by 3D Slicer (https://www.slicer.org/, Version 4.10.2). The CT data was available freely for research and educational use at the time of this study.
3D Slicer was employed to segment the thoracic bony structures using a radiodensity based threshold algorithm, which traces the bone based on the Hounsfield units. Due to a lack of contrast possibly from the poor resolution of the CT images, we were not able to segment the respective soft tissue layers using thresholding. Hence, the intercostal muscles were manually drawn with the paintbrush function. Intrathoracic organs were all removed to create a central cavity. From initial experimentation, we found that incision and dissection were too difficult to perform if the task trainer was printed at the true thoracic thickness. Hence, a decision was made to thin out the chest wall. At the 4th and 5th intercostal space midaxillary line, the mean chest wall thickness is 39mm (Laan et al., 2016), but our model measured at 18mm at this corresponding anatomical landmark.
Further processing was done to smoothen the contours of the model (see Appendix, A). Subsequently, the anatomical structures were saved as stereolithography (STL) file and exported into Materialise Magics (Version 20 by Materialise, Belgium).
On Magics, cut and Boolean techniques were used to create the replaceable component. This space was demarcated by the 5th to 6th intercostal space, between anterior axillary to the mid axillary line. To create a secure fit for the replaceable piece, a groove was created and reinforced using the cut and punch function which generates teething to maximise friction. The main frame measured 23cm (length) x 19.5cm (width) x 23.5cm (height), while the replaceable part measured 9cm (length x 8.1cm (width) x 0.8cm (height). The Fix Wizard and Shrink Wrap Part functions were used to repair the surface mesh and eliminate holes and loose shells. The models were then exported using IdeaMaker® (Raise3D, USA) and uploaded to the printer.
The model was printed in two parts: the main frame was printed using fusion deposition modelling (FDM). This technology extrudes a continuous filament of melted thermoplastic, repeated by layer based on the design coordinates. Bones were printed with polylactic acid (PLA) which is a rigid material while the intercostal muscles were printed with thermoplastic urethane (TPU) which is a flexible material. Support was printed using PLA. We utilised a dual nozzle extrusion printer (Raise3D Pro 2, Raise3D, USA) to allow us to print the bony and soft tissue simultaneously, thereby increasing convenience. The following settings were used: printing speeds were reduced to 25mm/s, retraction of the TPU extrusion head was disabled, nozzle temperatures were set at 200°C, and build plate temperature was at 65°C. Post-print processing was done to remove the support, with subsequent filing and sanding.
The replaceable part was printed using Objet500 Connex 3 (Stratasys Ltd, Eden Prairie, MN), a multi-material printer utilising material jetting technology. This technology drops liquid photopolymers onto the build tray and simultaneously cures the material using UV light. As such, we can mix plastic and rubber to create hybrid consistencies (Mogali et al., 2018) of varying shore hardness. Two materials were selected to achieve the desired haptics: VeroWhite (FullCure, RGD835) was the stiff plastic photopolymer used for bones, while Tango Plus (FullCure, 930) was the rubber photopolymer used for simulating soft tissue. Support resin (FullCure, 706) was also used for printing. Post-printing processing was required to remove the support resin.
Skin coloured silicone sheets of 5 mm thickness were wrapped around the model using generic superglue. The task trainer was cable tied to stainless steel supports and screwed onto a laminated wood baseplate. Cut sponges were wrapped in duct tape to simulate the lung parenchyma and placed into the central cavity created.
III. RESULTS
The completed task trainer is shown in Figure 1. Both the main frame and replaceable piece provided simulation for the ribs, intercostal muscles, and skin.
The 3D thoracostomy task trainer costs approximately SGD$130 (or USD$97) (excluding manpower and printer cost)–see Appendix, B). The baseplate and mount were repurposed and did not add to costs.
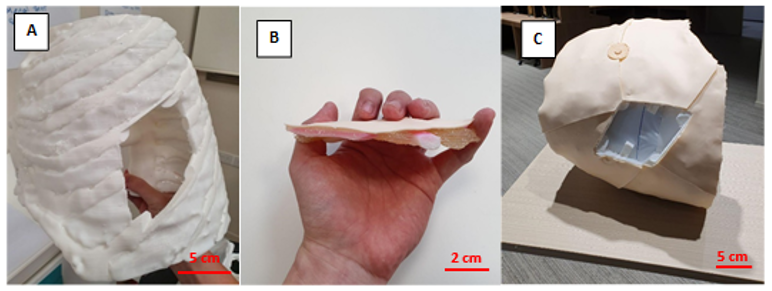
Note. A = completed hemithorax main frame using FDM printing; B= replaceable piece; C = task trainer without the replaceable piece. Figure 1. Photos of the completed task trainer
The main frame required 676g of polylactic acid and 114g of thermoplastic urethane. The replaceable piece required 30g of VeroWhite, 22g of Tango Plus, and 66g of Support706. It took a total of approximately 118 hours to print the entire task trainer.
IV. DISCUSSION
Our methodology addressed several issues with the model as outlined by the Brazilian team (Bettega et al., 2019). The proposed methodology here required less manual assembly of components, thereby saving time and improving fabrication. By utilising dual extrusion printing, construction was simplified while integrating an additional material for varying consistencies. The creation of a replaceable piece also meant long term savings in the cost of utilising this model. These logistical advantages would make it easier to adopt our proposed task trainer.
Secondly, simple materials such as foam pads and silicone sheets were inferior in simulating human tissue. Our utilisation of material jetting technology with the Objet500 Connex 3 (Stratasys Ltd, Eden Prairie, MN) printer allowed us to blend plastic and rubber materials to better recreate the consistency of human tissue. This technology and blend of materials have been extensively validated in other simulation models (Mogali et al., 2018).
Cost remains an important impedance to the widespread use of simulation in procedural education. We performed a surface comparison of our product against an existing commercial model in use by a local hospital in Singapore (LF03770U by Lifeform, NASCO, USA). The task trainer outlined here (~USD$97) is significantly cheaper than the commercial trainer (~USD$1,800). Also, our material blend provides superior haptics and bony structures in the replaceable component, as compared to a plain silicone insert in the Lifeform model. These should provide improvements in the quality and quantity of simulation opportunities for training physicians.
Unfortunately, we were not able to recreate the anatomical thickness of the thorax given our material limitations at the time of writing. This inaccurate depth of dissection creates a confounding variable when evaluating our task trainer against existing cadaveric or commercial simulators. Hence, an evaluation of this task trainer was withheld to address this limitation in our future prototype. Moving forward, we plan to invite physicians to validate the efficacy of our improved task trainer.
V. CONCLUSION
We have outlined the methodology for creating a 3D printed tube thoracostomy task trainer using a combination of printing technologies. The outlined task trainer could potentially provide superior haptics at a lower cost while improving fabrication. However, an equitable validation against an existing modality of simulation can only be done after we achieve a comparable anatomical fidelity.
In our continued search for sustainable simulation models, 3D printing shows great potential in reproducing anatomical detail with superior cost efficiency. The growing availability of 3D printing infrastructure makes the large-scale adoption of such task trainers ever more realistic. It makes it therefore worthwhile to invest in the creation of the perfect 3D printed task trainer.
Notes on Contributors
Mr. Wen Hao Chen is an undergraduate medical student with the Lee Kong Chian School of Medicine, Singapore. He was involved in the development of the task trainer, along with co-authoring the submitted manuscript.
Dr. Shairah Radzi is a research fellow with the Lee Kong Chian School of Medicine, Singapore. She was involved in the development of the task trainer, along with co-authoring the submitted manuscript.
Dr. Li Qi Chiu is a consultant physician in the Department of Emergency Medicine in Tan Tock Seng Hospital, Singapore. She was involved in the development of the task trainer, along with co-authoring the submitted manuscript.
Assoc. Prof Wai Yee Yeong is the Associate Chair (Students) of the School of Mechanical and Aerospace Engineering, Nanyang Technological University, Singapore. She was involved in the development of the task trainer, providing her technical expertise on the 3D printing process, along with co-authoring the submitted manuscript.
Asst. Prof Sreenivasulu Reddy Mogali is the Head of Anatomy and Principal Investigator in Clinical Anatomy and Medical Education at Lee Kong Chian School of Medicine, Singapore. He was involved in the development of the task trainer, along with co-authoring the submitted manuscript. He serves as the principal investigator.
Ethical Approval
Approved by Nanyang Technological University’s Institutional Review Board (2019-07-017). The CT scans used were anonymised and provided free for education and research use by 3D Slicer (https://www.slicer.org/, Version 4.10.2).
Acknowledgement
The authors thank the staff and faculty of the Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore for supporting this research; Singapore Centre for 3D Printing, Nanyang Technological University for their technical support.
Funding
This project was funded by the Ministry of Education Research Start-Up Grant, Lee Kong Chian School of Medicine, Nanyang Technological University Singapore.
Declaration of Interest
All authors declare no conflict of interest. The authors alone are responsible for the content and writing of the article.
References
Bettega, A. L., Brunello, L. F. S., Nazar, G. A., De-Luca, G. Y. E., Sarquis, L. M., Wiederkehr, H. de A., … Pimentel, S. K. (2019). Chest tube simulator: Development of low-cost model for training of physicians and medical students. Revista Do Colégio Brasileiro de Cirurgiões, 46(1). https://doi.org/10.1590/0100-6991e-20192011
Hernandez, M. C., El Khatib, M., Prokop, L., Zielinski, M. D., & Aho, J. M. (2018). Complications in Tube Thoracostomy: Systematic review and Meta-analysis. The Journal of Trauma and Acute Care Surgery, 85(2), 410–416. https://doi.org/10.1097/TA.0000000000001840
Kovacs, G., Levitan, R., & Sandeski, R. (2018). Clinical Cadavers as a Simulation Resource for Procedural Learning. AEM Education and Training, 2(3), 239–247. https://doi.org/10.1002/aet2.10103
Laan, D. V., Vu, T. D. N., Thiels, C. A., Pandian, T. K., Schiller, H. J., Murad, M. H., & Aho, J. M. (2016). Chest Wall Thickness and Decompression Failure: A Systematic Review and Meta-analysis Comparing Anatomic Locations in Needle Thoracostomy. Injury, 47(4), 797–804. https://doi.org/10.1016/j.injury.2015.11.045
Mogali, S. R., Yeong, W. Y., Tan, H. K. J., Tan, G. J. S., Abrahams, P. H., Zary, N., … Ferenczi, M. A. (2018). Evaluation by medical students of the educational value of multi-material and multi-colored three-dimensional printed models of the upper limb for anatomical education. Anatomical Sciences Education, 11(1), 54–64. https://doi.org/10.1002/ase.1703
*Sreenivasulu Reddy Mogali
11 Mandalay Road, Singapore 308232
Lee Kong Chian School of Medicine,
Nanyang Technological University
Email: sreenivasulu.reddy@ntu.edu.sg











